Calculate the work for the expansion of 3 moles of B,H; at 40°C from 2.74 L to 3.89 L using van der correction for intermolecular forces (a) is 6.048 bar L'/mol" and the adjustment factor of the volum The general equation for van der Waals is written below: RT A. 2352.2376 J B. -2352.2376 J C. 2118.1071J D. -2118.1071J
Calculate the work for the expansion of 3 moles of B,H; at 40°C from 2.74 L to 3.89 L using van der correction for intermolecular forces (a) is 6.048 bar L'/mol" and the adjustment factor of the volum The general equation for van der Waals is written below: RT A. 2352.2376 J B. -2352.2376 J C. 2118.1071J D. -2118.1071J
Physics for Scientists and Engineers, Technology Update (No access codes included)
9th Edition
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter21: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 21.25P
Related questions
Question
Use integration in answering this problem. Work is equal to the integral of pressure in terms of volume from V1 to V2
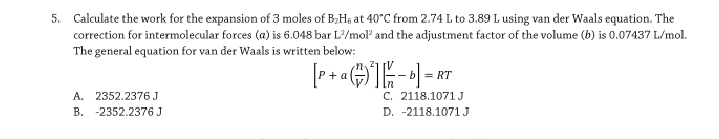
Transcribed Image Text:5. Calculate the work for the expansion of 3 moles of B;Hs at 40"C from 2.74 L to 3.89 L using van der Waals equation. The
correction for intermolecular forces (a) is 6.048 bar L?/mol and the adjustment factor of the volume (b) is 0.07437 L/mol.
The general equation for van der Waals is written below:
+ a
=.
A. 2352.2376J
B. -2352.2376 J
C. 2118.1071J
D. -2118.1071 J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning