Q: need correct answer within 30-40 mins I'll give you multiple upvote
A:
Q: 1,922 Joules of heat are added to 42.5 grams of water, initially at 20∘C. What is the final…
A: The final temperature is given below
Q: For a solution of formic acid, a weak acid with a pKa of 8.74, at what pH will the concentration of…
A: Using Henderson-Hasselbalch equation for buffer solution :- pH = pKa + log([conjugate base]/[weak…
Q: Consider the titration of 50.0 mL of 0.0500 M NaCl with 0.100 M AgNO3. Calculate pCl at various…
A: In a titration of NaCl with AgNO3 Silver chloride is precipitated and is known as…
Q: 4. Determine the standard enthalpy of formation for butane, 4 C(s, gr) + 5 H2(g) C4H10(g) using the…
A:
Q: The solubility of a gas is 0.980 at a pressure of 116 kPa. What is the solubility of the gas if the…
A: According to henry's law solubility increases when pressure increases....
Q: For the reaction A+3B 2C, the rate of disappearance of B given by (A[B]/At) may also be expressec…
A: The correct answer is given below
Q: QUESTION 7 a) Sketch all the steps required for the following synthesis. но. Compound H b) Write the…
A:
Q: Use the information below to make a written response question 2 RO3 (aq) + 12 H* (aq) + 3 M (s) 5 RO…
A:
Q: Gallium-67 is used medically in tumor-seeking agents. The half-life of gallium-67 is 78.2 hours. If…
A:
Q: Complete the Venn diagram below. Write down the similarities (at the middle) and differences (side)…
A: Soap :- i. Soaps work well in soft water. ii. Soaps cannot perform a cleansing action. iii.…
Q: Why does diffusion in solids important?
A: The correct answer about diffusion in solids is given below
Q: 2. Juan subjected a 10 L gas at STP with doubled temperature. What will happen te the final V? A.…
A: 2. Given that - Volume of gas = 10 L Temperature at STP = 273 K Pressure at STP = 1 Atm Final…
Q: Determine if the following proposed mechanisms has a rate law that is consistent with the observed…
A: Rate law of a reaction is written as product of concentration of reactants raised to their…
Q: Help needed with assigning the peak signals and determining the J values of this Isoamyl Acetate…
A: Coupling constant is denated by letter 'J' is the distance between two lines in multiplet. The unit…
Q: (+) i) Vs (+) В A ii) CF3 +) CF3 +) Vs H2N H2N A B
A: 1)
Q: Complete the balanced chemical equation for the following weak base and a strong acid. NaC2H3O2…
A: The unbalanced reaction is: NaC2H3O2aq+HNO3aq→
Q: If you want to reduce Sn* to Sn* and you have Fe", Fe, Al (s), and Al3+ available. What chemical…
A:
Q: Consider a solution made by massing 8.3 g of a nonvolatile solute (molar mass = 68.51 g/mol) and…
A: This question is related to the basics of solution and the different ways of expressing the…
Q: 17.A balloon filled with 3.0 L of He at 210 K and 2.00 atm is slowly cooled to 70 K under isobaric…
A:
Q: n compounds are aromatic? (A) (B) (C) (D) (E) (F) B. (G) (H) (1) H.
A:
Q: A Но D .CI 1-ВНСу2, THF 2* H2O2, NaOH
A: #A: Here the reactant is a ketone, and the product is an alcohol which is a reduced form of ketone.
Q: 4. Which of the foillowing examples demonstrates Charles' Law? A. hot air balloon B. lungs 5. If…
A:
Q: Direction: Try to spot the hidden words in the puzzle. The mystery words are associated with the…
A: Answers of the above puzzle are given in image below
Q: iven the following data, determine the (a) order of reaction of reactant A, (b) order of reaction of…
A:
Q: Complete the following reactions with the missing major products, starting reagents or reaction…
A: Carbonyl groups are reduced to alcohol by the reducing agents like LiAlH4, NaBH4. Olefins are are…
Q: A 1.876 g of the sample containing H2C2O4 requires 38.84 mL of 0.1032 M of NaOH for titration:…
A: Answer: This question is based on acid-base titration where acid is the analyte and base is the…
Q: Give a clear handwritten answer..and give the mechanism of given bleow reaction
A:
Q: QUESTION 7 a) Sketch all the steps required for the following synthesis. он Compound H b) Write the…
A: Given is organically synthesis reaction.
Q: A solution of potassium permanganate is standardized using pure As2O3as the primary standard (upon…
A: we are required to find the molarity of the potassium permanganate.
Q: For each of the following, provide the product or reagent as necessary H20 NaBH4 + Hg(OAc)2 ELOH а)…
A:
Q: А 1) mCPBA 2) H30* В Br2, MeOH Но. HNO3 "Ме H2SO4
A:
Q: Acid dissociation constants for sulfurous acid are given below. Solution A and Solution B are mixed.…
A: Given data,Molarity of KHSO3=1.00MVolume of KHSO3=200mLMolarity of KOH=1.00MVolume of KOH=40mL
Q: Rank the following aromatic compounds in increasing order of reactivity toward Br2 (least reactive…
A: Bromination of benzene in presence of Lewis acid catalyst takes place aromatic electrophilic…
Q: 6. Which gas is least dense at 1.03 atm and 301 K? a. CO2 b. Ne c. N2 d. Хe 7. A 1.2 L sample of an…
A: To find which gas is least dense, we have to use ideal gas equation i.e. PV = nRT
Q: Balance the following redox reaction in acldlc solution. 2+ Br (aq)+MnO,(aq) Br,(1)+Mn (aq) Br (aq)…
A: The given redox reaction is as follows: Br- + MnO4- → Br2 + Mn2+ A redox reaction is balanced by…
Q: What is the binding energy in kJ/mol La for lanthanum-139? |k]/mol 57;H+ 82;n The required masses…
A: 521H1 + 82 1n0 --->
Q: Which of the following are necessary steps in performing paper chromatography? I. Saturate the…
A: In paper chromatography the necessary conditions are 1. The chamber should be saturated with the…
Q: For the chemical reaction system described by the diagram below, which statement is true? Energy…
A: The given diagram is an energy versus reaction progress graph. From this graph we have to conclude…
Q: A sizeable uranium deposit was found in northern Canada. When one kilogram of the uranium ore was…
A: Know Initial amount of uranium ore (a) = 1000 g Half life of uranium = 4,510,000,000 yrs Amount of…
Q: 19-1 Review A harmonic oscillator is vibrating at a frequency of 6.11x1014s. Use the harmonic…
A:
Q: What Volume of the titrant is needed to reach the End Point having 5g of sample and 1N H2SO4 as a…
A: According to law of equivalence, an acid exactly neutralizes the same number of equivalents of base…
Q: Explain why salts can be acidic, basic, or neutral.
A: Salt is formed by the reaction of acid and base.
Q: then Br then Br Br
A: Solution: We know alpha hydrogen to the any carbonyl group are acidic. If there are two different…
Q: What is the equation to determine cell voltages using standard reduction potential? 2. What are the…
A:
Q: Ј.B. is a 52-year-old man weighing 72 kg who has been treated with gentamicin for confirmed…
A: According to the pharmacokinetics, we will need the following formulae to solve the given problem :…
Q: Draw the major product formed in the reaction. of NaOH, H20 A
A:
Q: 1) H2O2 (iii) 2) PC13 3) H2O ČOOH
A:
Q: Based on the reaction below, what is the correct K, expression for the this complex ion? 3+ Co3+ +…
A: Kf for equilibrium reaction is :- Kf = [product]÷[reactant]
Q: The anode compartment of a galvanic cell consists of a Zn anode immersed in 0.05M ZnCl2 The cathode…
A:
Can you make a conclusion for this table? Explaining how it gets the answer.
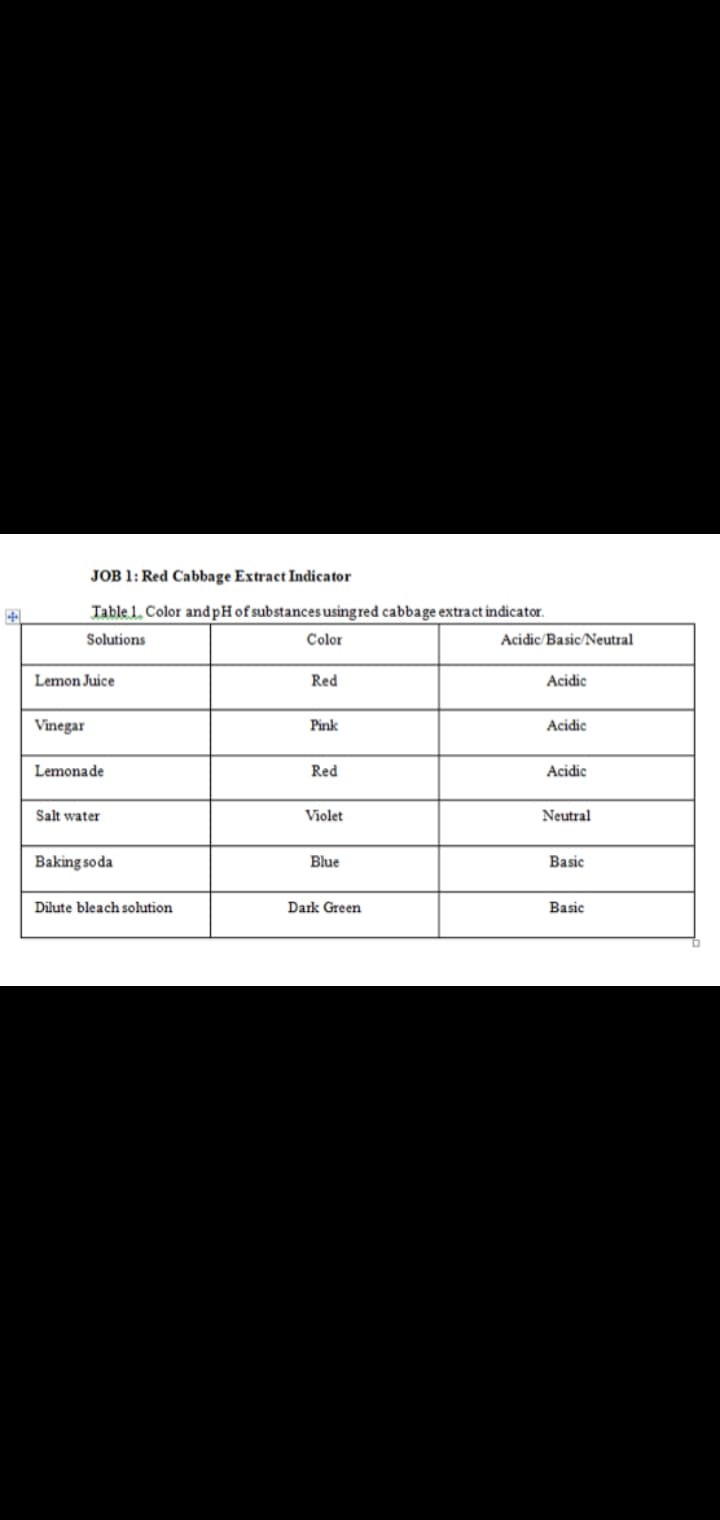
Step by step
Solved in 2 steps

- Prepared buffer solution GivenAmmonia Volume = 68 mL | Ammonia Concentration = 0.17 MAmmonium Chloride Volume = 42 mL | Ammonium Chloride Concentration = 0.13 MDissociation Constant of Ammonia: 1.8x10-5What is the total vol. of bufferHow to write the methodology? I have a practical of the MEASUREMENT USING PH METER, I don't know how to write the methodology. Here is the introduction: A pH meter is an electronic device mainly used for qualitative measurement, for thedetermination of acid and the basic value of a solution. Generally, it measures thehydrogen ion concentration/activity [H+] in a solution. It is denoted as:pH = - log10 [H+]In a pure water solution, the concentration of [H+] and [OH-] ions, respectively, rangefrom 1.0 x 10-1 M to 1.0 x 10-14 M. When [H+] is equal to [OH-] as when pure waterdissociates, the hydrogen ion concentration of pure water is equal to 1.0 x 10-7 M orpH = 7.00, defined as a neutral solution at 25°C, to be of temperaturedependent/endothermic dissociation.[H+] = [OH-] = 1.0 x 10-7 MWhen an ionic or polar substance is dissolved in water, it may change the relativenumbers of H+ and OH-. The higher the pH number, the lower the hydrogen ion concentration, and vice versa. Solution with an…Create a schemantic diagram of qualitative analysis for CoCO3.
- Prepared buffer solution GivenAmmonia Volume = 68 mL | Ammonia Concentration = 0.17 MAmmonium Chloride Volume = 42 mL | Ammonium Chloride Concentration = 0.13 MDissociation Constant of Ammonia: 1.8x10-5What is the final concentration of the baseEach indicator gradually changes colour over a range of about how many pH units?Table 12.5 (data) Indicator Color in 0.05 M HCl Color in 0.05 M NaOH Methyl red Bromcresol Phenolphthalein Methyl orange Methyl violet Table 12.6 (report) Indicator showing very distinct color difference in acid and base Indicator showing only slight color difference in acid and base
- Phenolphthalein indicator is a weak acid with Ka = 10-9 mol/dm-3. It is colourless while its conjugate base is pink in solution. Define the term" WEAK ACID"The acid-base indicator ethyl orange turns from red to yel-low over the pH range 3.4 to 4.8. Estimate Kₐ for ethyl orange.An acid-base indicator is usually a weak acid with a characteristic color in the protonated and deprotonated forms. Because bromocresol green is an acid, it is convenient to represent its rather complex formula as HBCG. HBCG ionizes in water according to the following equation: HBCG + H2O ⇌ BCG- + H3O+ (yellow) (blue) a. Write the Ka expression for bromocresol green based on the equation above. b. When [BCG-] = [HBCG], then show that the expression simplifies to Ka = [H3O+]. If you know the pH of the solution, then the [H3O+] and Ka can be determined. c. What would be the color of the solution if there were equal concentrations of HBCG and BCG-?
- In the experiment, we used titration to determine the total acid content ofsamples, which we reported in terms of molarity. Reporting acidity through pHmeasurements is quite different, in that we can only measure the amount of theacid in its ionized form. You were tasked to investigate a clear aqueous solutionof an unknown monoprotic acid. You decided to use two methods togather data.Method 1 – TITRATION: A 10. mL aliquot of the sample was diluted with 25 mLdistilled water. Two drops of phenolphthalein were added and then it was titrated3.54 mL of 0.048 M standardized NaOH to the endpoint.Method 2 – pH STRIP: You took 1 mL of the sample and used a pH strip toestimate the pH, which turned out to be around 3.3. Another 1 mL of the samplewas diluted with 9 mL of water. The pH was taken again and is now around 3.8.a) Calculate the molarity of the acid using the titration data.b) If we assume that the titrated unknown is a strong acid, predict thepH of the sample.c) Using pH strip results,…100ml of asample of water required 10 ml of N/10 acid for titrating using phenolphthalein indicator. A100 ml sample of water was again taken and methyl orange was used as indicator, when15ml of N/10 acid was required for neutralization. Interpret the results of alkalinities in ppm.A professor prepares a buffer solution that they need for the purification of protein from human cell lysates. They did mix weak acid and conjugate base and obtained the initial solution, which is characterised by these parameters. Final volume: 200mltotal buffer compound concentration: 150mMIntitial concentration weak acid: 0.03MInitial concentration conjugate base: 0.12MInitial pH 6.9buffer compound pka 6.3



