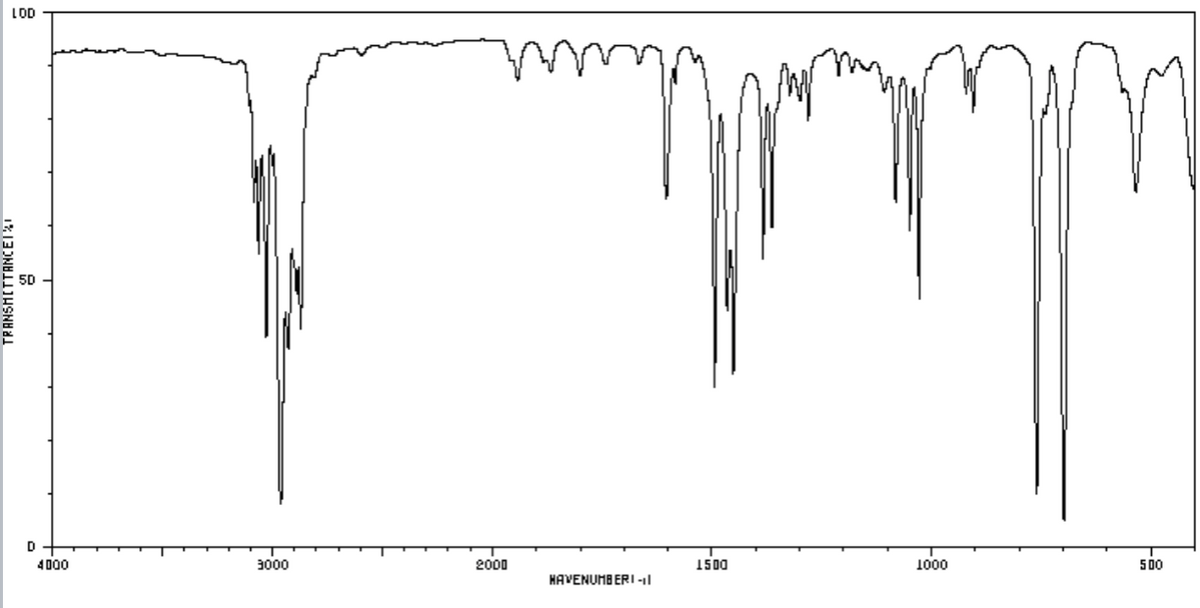
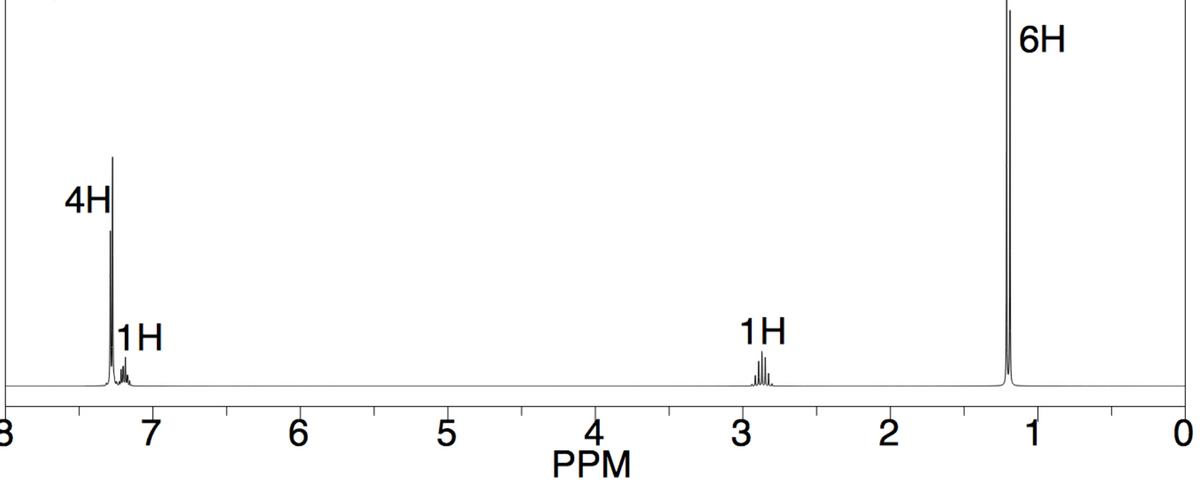
Carbon has a percent composition of 89.9%. Hydrogen has a percent compositon of 10.1%. The percent composition of oxygen or any other element will not be given, but it may still be in the formula. Based on this information along with the ir and nmr spectra come up with an empirical formula and show your steps.
Carbon has a percent composition of 89.9%. Hydrogen has a percent compositon of 10.1%. The percent composition of oxygen or any other element will not be given, but it may still be in the formula. Based on this information along with the ir and nmr spectra come up with an empirical formula and show your steps.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter3: Measurement And Chemical Calculations
Section: Chapter Questions
Problem 5E
Related questions
Question
a) Carbon has a percent composition of 89.9%. Hydrogen has a percent compositon of 10.1%. The percent composition of oxygen or any other element will not be given, but it may still be in the formula. Based on this information along with the ir and nmr spectra come up with an empirical formula and show your steps.
b) Once you have the formula, analyze the spectra again and draw the correct structure and label the hydrogens found in different environments as A, B, C etc. Then make an NMR data table which includes the labeled hydrogens, proton chemical shift, integration, splitting pattern and what it couples to (see example table below):
Example of what should be included in the table:
- the nmr table should include (in the example table imagine the structure of ethanol is drawn with the CH3 hydrogens labeled as A, the CH2 hydrogens labeled as B, and the OH hydrogen labeled as C) :
| hydrogen | proton chemical shift | integration | splitting pattern | couples to.. |
| A | 1.2 | 3 | triplet | B |
| B | 3.7 | 2 | quartet | A |
| C | 2.6 | 1 | singlet | - |
Transcribed Image Text:001
TRANSMITTANCET
D
4000
3000
2000
mm
HAVENUMBERI-I
1500
1000
500
Transcribed Image Text:3
4H
1H
7
6
5
4
PPM
1H
3
2
6H
1
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning