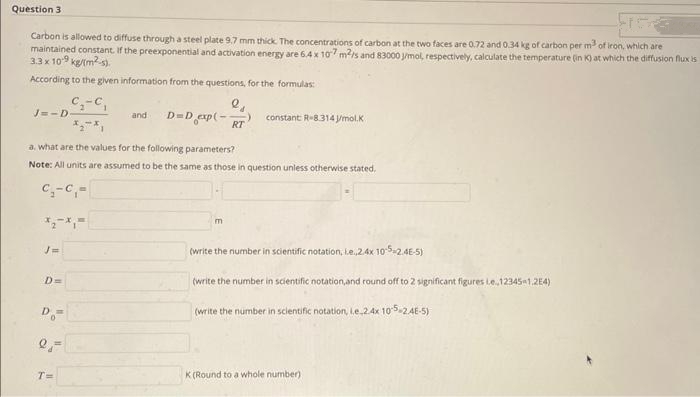
Carbon is allowed to diffuse through a steel plate 9.7 mm thick. The concentrations of carbon at the two faces are 0.72 and 0.34 kg of carbon per m³ of iron, which are maintained constant. If the preexponential and activation energy are 6.4 x 107 m2/s and 83000 j/mol, respectively, calculate the temperature (in K) at which the diffusion flux is 3.3 x 109 kg/(m²-s). According to the given information from the questions, for the formulas: C₂-C₁ J=-D- J= D= a. what are the values for the following parameters? Note: All units are assumed to be the same as those in question unless otherwise stated. C₁-C₁- Do- 25 T= 2₁ RT and D=D exp(-7 m constant R-8.314 J/mol.K (write the number in scientific notation, i.e.,2.4x 10-5-2.4E-5) (write the number in scientific notation, and round off to 2 significant figures Le.,12345-1,2E4) (write the number in scientific notation, i.e.2.4x 10-5-2.4E-5) K (Round to a whole number)
Carbon is allowed to diffuse through a steel plate 9.7 mm thick. The concentrations of carbon at the two faces are 0.72 and 0.34 kg of carbon per m³ of iron, which are maintained constant. If the preexponential and activation energy are 6.4 x 107 m2/s and 83000 j/mol, respectively, calculate the temperature (in K) at which the diffusion flux is 3.3 x 109 kg/(m²-s). According to the given information from the questions, for the formulas: C₂-C₁ J=-D- J= D= a. what are the values for the following parameters? Note: All units are assumed to be the same as those in question unless otherwise stated. C₁-C₁- Do- 25 T= 2₁ RT and D=D exp(-7 m constant R-8.314 J/mol.K (write the number in scientific notation, i.e.,2.4x 10-5-2.4E-5) (write the number in scientific notation, and round off to 2 significant figures Le.,12345-1,2E4) (write the number in scientific notation, i.e.2.4x 10-5-2.4E-5) K (Round to a whole number)
Related questions
Question
Transcribed Image Text:Question 3
which are
Carbon is allowed to diffuse through a steel plate 9.7 mm thick. The concentrations of carbon at the two faces are 0.72 and 0.34 kg of carbon per m³ of iron,
maintained constant. If the preexponential and activation energy are 6.4 x 107 m²/s and 83000 J/mol, respectively, calculate the temperature (in K) at which the diffusion flux is
3.3 x 10-9 kg/(m²).
According to the given information from the questions, for the formulas:
J=-D-
D=
C₂-C
Do=
2 =
-C₁
T=
and
a. what are the values for the following parameters?
Note: All units are assumed to be the same as those in question unless otherwise stated.
C₁-C₁-
e₁
D=D_exp(- RT
constant R-8.314 J/mol.K
m
(write the number in scientific notation, le.,2.4x 10-5-2.4E-5)
(write the number in scientific notation, and round off to 2 significant figures Le..12345-1,2E4)
(write the number in scientific notation, i.e.2.4x 10-5-2.4E-5)
K(Round to a whole number)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images
