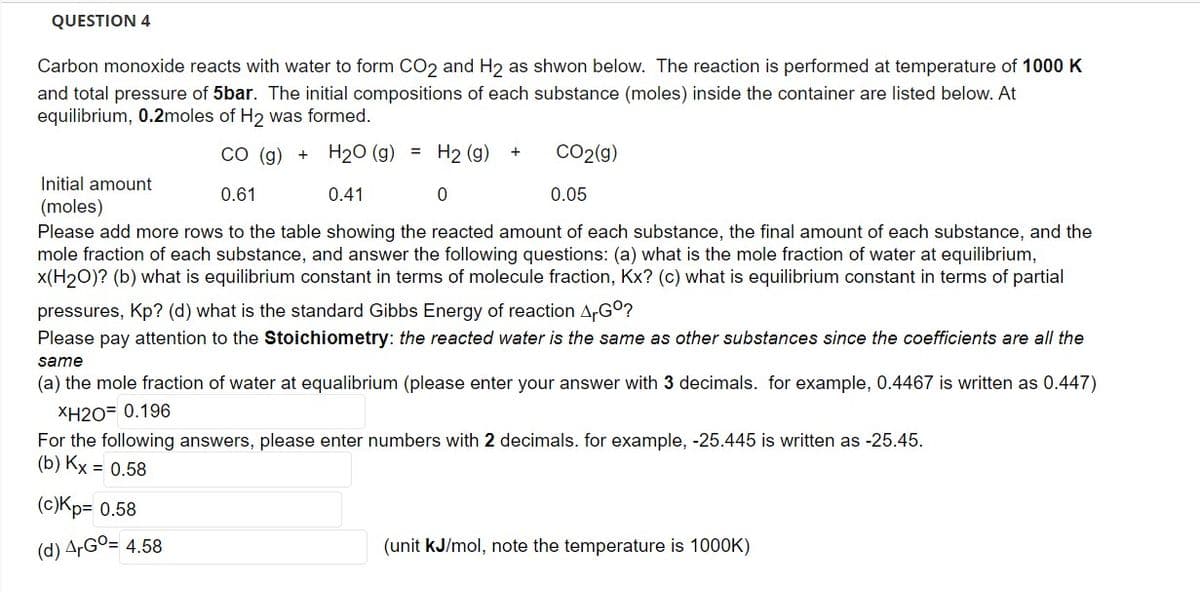
Carbon monoxide reacts with water to form CO2 and H₂ as shwon below. The reaction is performed at temperature of 1000 K and total pressure of 5bar. The initial compositions of each substance (moles) inside the container are listed below. At equilibrium, 0.2moles of H2 was formed. CO (g) + H₂O (g) = H2 (g) Initial amount (moles) 0.61 0.41 0 0.05 Please add more rows to the table showing the reacted amount of each substance, the final amount of each substance, and the mole fraction of each substance, and answer the following questions: (a) what is the mole fraction of water at equilibrium, x(H₂O)? (b) what is equilibrium constant in terms of molecule fraction, Kx? (c) what is equilibrium constant in terms of partial pressures, Kp? (d) what is the standard Gibbs Energy of reaction ArGº? Please pay attention to the Stoichiometry: the reacted water is the same as other substances since the coefficients are all the same (a) the mole fraction of water at equalibrium (please enter your answer with 3 decimals. for example, 0.4467 is written as 0.447) XH2O= 0.196 For the following answers, please enter numbers with 2 decimals. for example, -25.445 is written as -25.45. (b) Kx = 0.58 (c)Kp= 0.58 (d) ArGo= 4.58 + CO2(g) (unit kJ/mol, note the temperature is 1000K)
Carbon monoxide reacts with water to form CO2 and H₂ as shwon below. The reaction is performed at temperature of 1000 K and total pressure of 5bar. The initial compositions of each substance (moles) inside the container are listed below. At equilibrium, 0.2moles of H2 was formed. CO (g) + H₂O (g) = H2 (g) Initial amount (moles) 0.61 0.41 0 0.05 Please add more rows to the table showing the reacted amount of each substance, the final amount of each substance, and the mole fraction of each substance, and answer the following questions: (a) what is the mole fraction of water at equilibrium, x(H₂O)? (b) what is equilibrium constant in terms of molecule fraction, Kx? (c) what is equilibrium constant in terms of partial pressures, Kp? (d) what is the standard Gibbs Energy of reaction ArGº? Please pay attention to the Stoichiometry: the reacted water is the same as other substances since the coefficients are all the same (a) the mole fraction of water at equalibrium (please enter your answer with 3 decimals. for example, 0.4467 is written as 0.447) XH2O= 0.196 For the following answers, please enter numbers with 2 decimals. for example, -25.445 is written as -25.45. (b) Kx = 0.58 (c)Kp= 0.58 (d) ArGo= 4.58 + CO2(g) (unit kJ/mol, note the temperature is 1000K)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section8.6: Gas Mixtures And Partial Pressures
Problem 8.10PSP
Related questions
Question
4
hell0 is this correct
Transcribed Image Text:QUESTION 4
Carbon monoxide reacts with water to form CO2 and H2 as shwon below. The reaction is performed at temperature of 1000 K
and total pressure of 5bar. The initial compositions of each substance (moles) inside the container are listed below. At
equilibrium, 0.2moles of H₂ was formed.
CO (g) + H₂O (g) = H2 (g) +
Initial amount
(moles)
0.61
0.41
0.05
Please add more rows to the table showing the reacted amount of each substance, the final amount of each substance, and the
mole fraction of each substance, and answer the following questions: (a) what is the mole fraction of water at equilibrium,
x(H₂O)? (b) what is equilibrium constant in terms of molecule fraction, Kx? (c) what is equilibrium constant in terms of partial
pressures, Kp? (d) what is the standard Gibbs Energy of reaction Gº?
Please pay attention to the Stoichiometry: the reacted water is the same as other substances since the coefficients are all the
same
(a) the mole fraction of water at equalibrium (please enter your answer with 3 decimals. for example, 0.4467 is written as 0.447)
XH2O= 0.196
For the following answers, please enter numbers with 2 decimals. for example, -25.445 is written as -25.45.
(b) Kx = 0.58
(c)Kp= 0.58
(d) ArGo= 4.58
0
CO2(g)
(unit kJ/mol, note the temperature is 1000K)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning