Cell membranes are semipermeable, and osmosis is an ongoing process in the body. Differences in osmotic pressure inside and outside a red blood cell can result in crenation (shriveling) or hemolysis (rupture). The diagram shows three solutions: an isotonic solution, a hypertonic solution, and a hypotonic solution. Place the appropriate red blood cell in each solution. isotonic solution hypertonic solution hypotonic solution Answer Bank ww*****E*S isotonic solution hypertonic solution hypotonic solution Answer Bank Given that blood exerts the same osmotic pressure as a 0.15 M NaCl solution, which solution could be the hypertonic solution? 0.008 M NaCl solution O 0.15 M NaCl solution O 0.68 M NaCl solution
Cell membranes are semipermeable, and osmosis is an ongoing process in the body. Differences in osmotic pressure inside and outside a red blood cell can result in crenation (shriveling) or hemolysis (rupture). The diagram shows three solutions: an isotonic solution, a hypertonic solution, and a hypotonic solution. Place the appropriate red blood cell in each solution. isotonic solution hypertonic solution hypotonic solution Answer Bank ww*****E*S isotonic solution hypertonic solution hypotonic solution Answer Bank Given that blood exerts the same osmotic pressure as a 0.15 M NaCl solution, which solution could be the hypertonic solution? 0.008 M NaCl solution O 0.15 M NaCl solution O 0.68 M NaCl solution
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter8: Solutions
Section: Chapter Questions
Problem 8.113EP: Consider two solutions, A and B, separated by an osmotic semipermeable membrane that allows only...
Related questions
Question
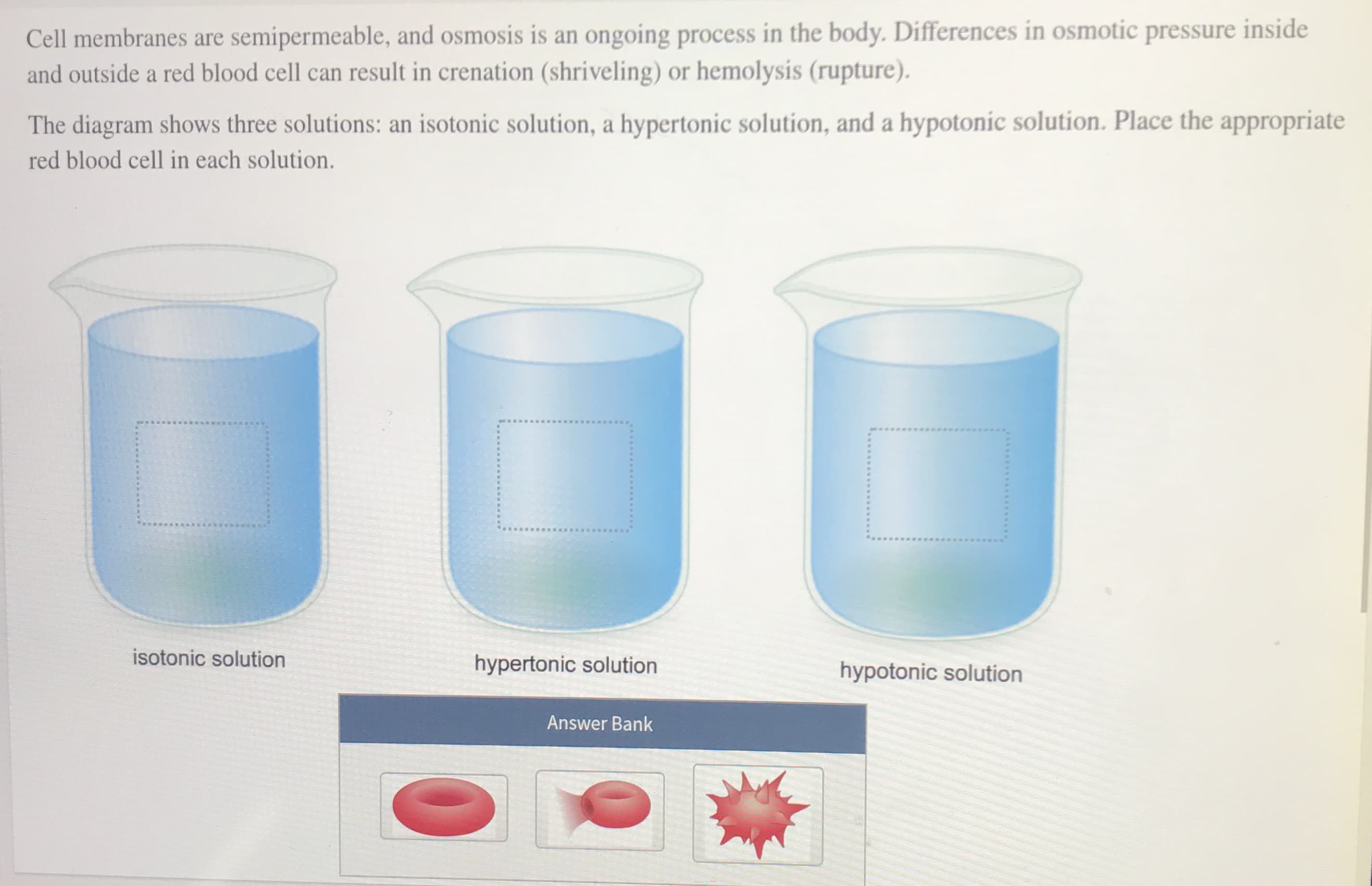
Transcribed Image Text:Cell membranes are semipermeable, and osmosis is an ongoing process in the body. Differences in osmotic pressure inside
and outside a red blood cell can result in crenation (shriveling) or hemolysis (rupture).
The diagram shows three solutions: an isotonic solution, a hypertonic solution, and a hypotonic solution. Place the appropriate
red blood cell in each solution.
isotonic solution
hypertonic solution
hypotonic solution
Answer Bank
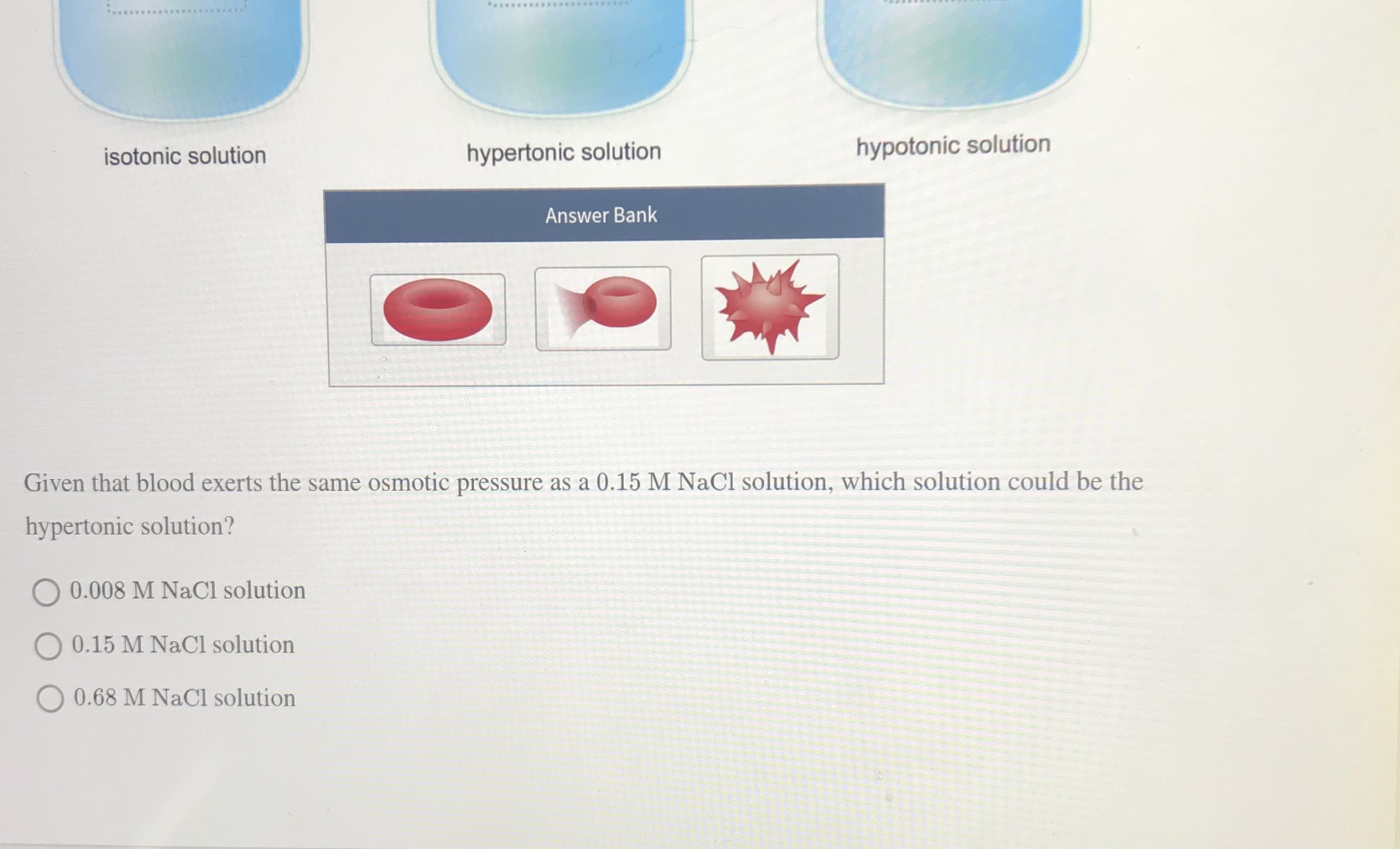
Transcribed Image Text:ww*****E*S
isotonic solution
hypertonic solution
hypotonic solution
Answer Bank
Given that blood exerts the same osmotic pressure as a 0.15 M NaCl solution, which solution could be the
hypertonic solution?
0.008 M NaCl solution
O 0.15 M NaCl solution
O 0.68 M NaCl solution
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
