Centretor Foundaon Sedies in ciente (i) HBr (vi) PH3 (ii) HOBR (iii) NO (iv) NO* 9. (а). What is the change in hybridization of the central atoms in the reactants to the products (as underlined) in the following reactions? (b). Draw the the structures of the reactants and the products according to the hybridization theory. (i) BF3 + F BF4 (ii). PCIS + Cl→ PC16 Draw the hybridization orbital overlap for the following molecules. Label the bond angles between the bonds. 10. H. (i) C-N-H H. H. 11. For the following species, (i) (ii) State the orbital hybrid for the atoms labelled 1,2,3,4. State the bond angles labelled a,b,c. and state
Centretor Foundaon Sedies in ciente (i) HBr (vi) PH3 (ii) HOBR (iii) NO (iv) NO* 9. (а). What is the change in hybridization of the central atoms in the reactants to the products (as underlined) in the following reactions? (b). Draw the the structures of the reactants and the products according to the hybridization theory. (i) BF3 + F BF4 (ii). PCIS + Cl→ PC16 Draw the hybridization orbital overlap for the following molecules. Label the bond angles between the bonds. 10. H. (i) C-N-H H. H. 11. For the following species, (i) (ii) State the orbital hybrid for the atoms labelled 1,2,3,4. State the bond angles labelled a,b,c. and state
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter9: Bonding And Molecular Structure: Orbital Hybridization And Molecular Orbitals
Section: Chapter Questions
Problem 50GQ
Related questions
Question
Question 9.(b)
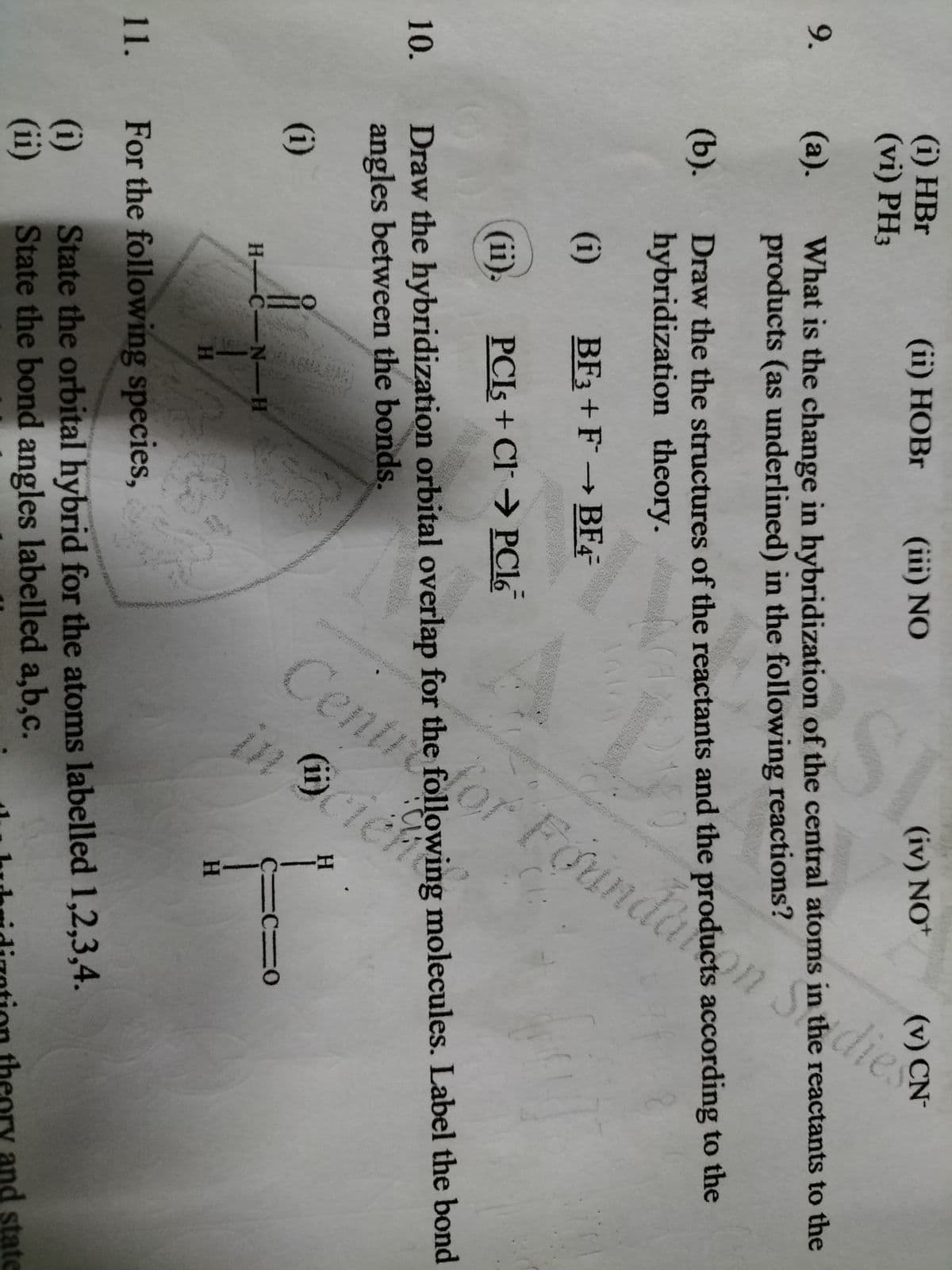
Transcribed Image Text:dlor Foundakon S
Cer
Founde
dies
(i) HBr
(vi) PH3
(ii) НОBr
(iii) NO
(iv) NO+
(v) CN-
9.
(а).
What is the change in hybridization of the central atoms in the reactants to the
products (as underlined) in the following reactions?
(b).
Draw the the structures of the reactants and the products according to the
hybridization theory.
(i)
BF3 + F
→ BF4-
(ii).
PCIS + Cl→ PC16
Draw the hybridization orbital overlap for the following molecules. Label the bond
angles between the bonds.
10.
(i)
(ii)
H
H C-N-H
HA
H.
11. For the following species,
(i)
(ii)
State the orbital hybrid for the atoms labelled 1,2,3,4.
State the bond angles labelled a,b,c.
ory and state
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning