CH3 OH H3C `CH3 2-isopropyl-5-methylcyclohexanol There are four cis,trans isomers for 2-isopropyl-5-methylcyclohexanol, where the cis,trans designations of the substituents are made relative to the OH group: • up, up, up (cis,cis) • up, up, down (cis,trans) • up, down, down (trans,cis) • up, down, up (trans,trans) Consider the most stable chair for each of these isomers, and then draw the most stable and least stable isomer based on a comparison of the best chair for each one. • To simplify matters, consider only axial vs equatorial energies of the groups (i.e. do not worry about the interactions between the groups, which in reality is also important). • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • You do not have to explicitly draw H atoms. Use “flat" representations of rings, not chairs, in your drawing.
CH3 OH H3C `CH3 2-isopropyl-5-methylcyclohexanol There are four cis,trans isomers for 2-isopropyl-5-methylcyclohexanol, where the cis,trans designations of the substituents are made relative to the OH group: • up, up, up (cis,cis) • up, up, down (cis,trans) • up, down, down (trans,cis) • up, down, up (trans,trans) Consider the most stable chair for each of these isomers, and then draw the most stable and least stable isomer based on a comparison of the best chair for each one. • To simplify matters, consider only axial vs equatorial energies of the groups (i.e. do not worry about the interactions between the groups, which in reality is also important). • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • You do not have to explicitly draw H atoms. Use “flat" representations of rings, not chairs, in your drawing.
Chapter4: Organic Compounds: Cycloalkanes And Their Stereochemistry
Section4.SE: Something Extra
Problem 62AP: Alcohols undergo an oxidation reaction to yield carbonyl compounds on treatment with CrO3. For...
Related questions
Question
so pls draw the flat representation, not the Chair form of the overall Most stable and least stable forms
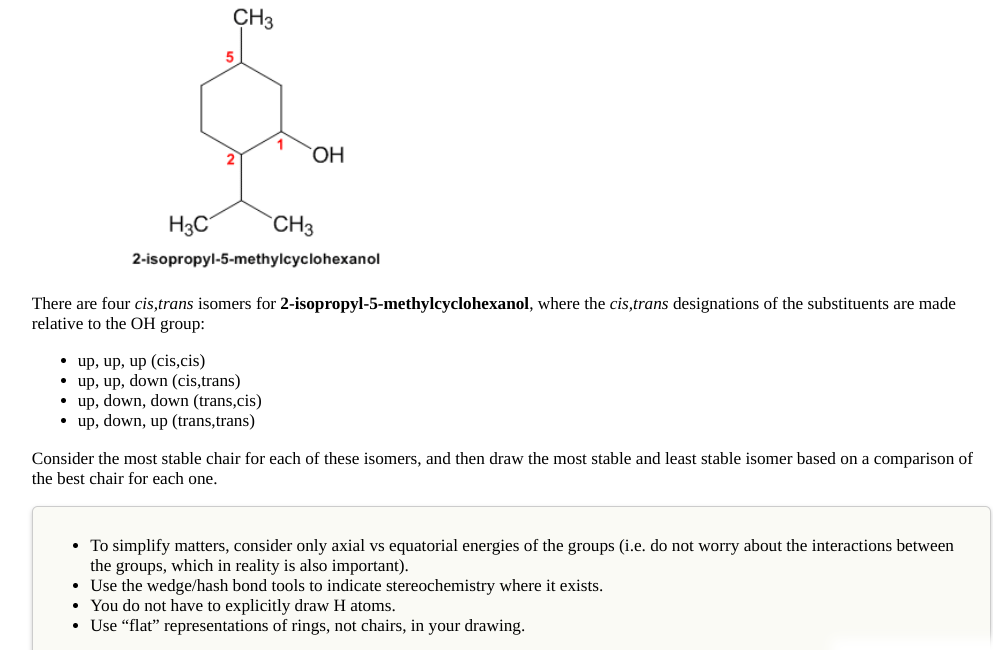
Transcribed Image Text:CH3
5
OH
H3C
`CH3
2-isopropyl-5-methylcyclohexanol
There are four cis,trans isomers for 2-isopropyl-5-methylcyclohexanol, where the cis,trans designations of the substituents are made
relative to the OH group:
• up, up, up (cis,cis)
• up, up, down (cis,trans)
• up, down, down (trans,cis)
• up, down, up (trans,trans)
Consider the most stable chair for each of these isomers, and then draw the most stable and least stable isomer based on a comparison of
the best chair for each one.
• To simplify matters, consider only axial vs equatorial energies of the groups (i.e. do not worry about the interactions between
the groups, which in reality is also important).
• Use the wedge/hash bond tools to indicate stereochemistry where it exists.
• You do not have to explicitly draw H atoms.
• Use “flat" representations of rings, not chairs, in your drawing.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning