Chapter4: Organic Compounds: Cycloalkanes And Their Stereochemistry
Section4.3: Stability Of Cycloalkanes: Ring Strain
Problem 8P: Each H↔H eclipsing interaction in ethane costs about 4.0 kJ/mol. How many such interactions are...
Related questions
Question
A proton transfer reaction can occur when an
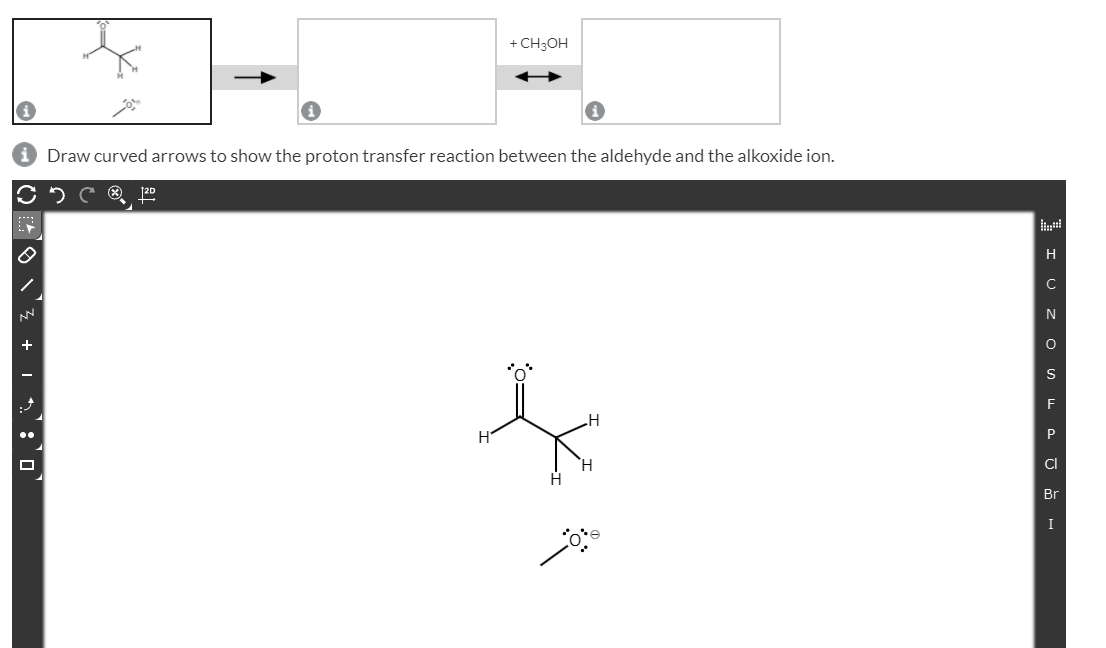
Transcribed Image Text:+ CH3OH
Draw curved arrows to show the proton transfer reaction between the aldehyde and the alkoxide ion.
120
H
+
S
F
P
H.
CI
Br
I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

