Chapter 08 Problem 4 r 4 of 27 Review I Constants I Periodic Table Charles's law states that the volume of a gas related to the absolute temperature when there is no change in the pressure or amount of gas: (Figure 1) is directly Part B Vi V2 What Celsius temperature, T2, is required to change the volume of the gas sample in Part A (T 9.0 ° C, V 1.53x103 L) to a volume of 3.06x10 L? Assume no change in pressure or the amount of gas in the balloon. T3 Ti Express your answer with the appropriate units. Figure < > 1 of 1 View Available Hint(s) ? °C T2= 0.06 Previous Answers Submit X Incorrect; Try Again; One attempt remaining Higher temperature Larger volume Lower temperature Smaller volume Next> Provide Feedback P Pearson Copyright O 2019 Pearson Education Inc. All rights reserved.| Terms of Use | Privacy Policy | Permissions | Contact Us का
Chapter 08 Problem 4 r 4 of 27 Review I Constants I Periodic Table Charles's law states that the volume of a gas related to the absolute temperature when there is no change in the pressure or amount of gas: (Figure 1) is directly Part B Vi V2 What Celsius temperature, T2, is required to change the volume of the gas sample in Part A (T 9.0 ° C, V 1.53x103 L) to a volume of 3.06x10 L? Assume no change in pressure or the amount of gas in the balloon. T3 Ti Express your answer with the appropriate units. Figure < > 1 of 1 View Available Hint(s) ? °C T2= 0.06 Previous Answers Submit X Incorrect; Try Again; One attempt remaining Higher temperature Larger volume Lower temperature Smaller volume Next> Provide Feedback P Pearson Copyright O 2019 Pearson Education Inc. All rights reserved.| Terms of Use | Privacy Policy | Permissions | Contact Us का
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 78QAP
Related questions
Question
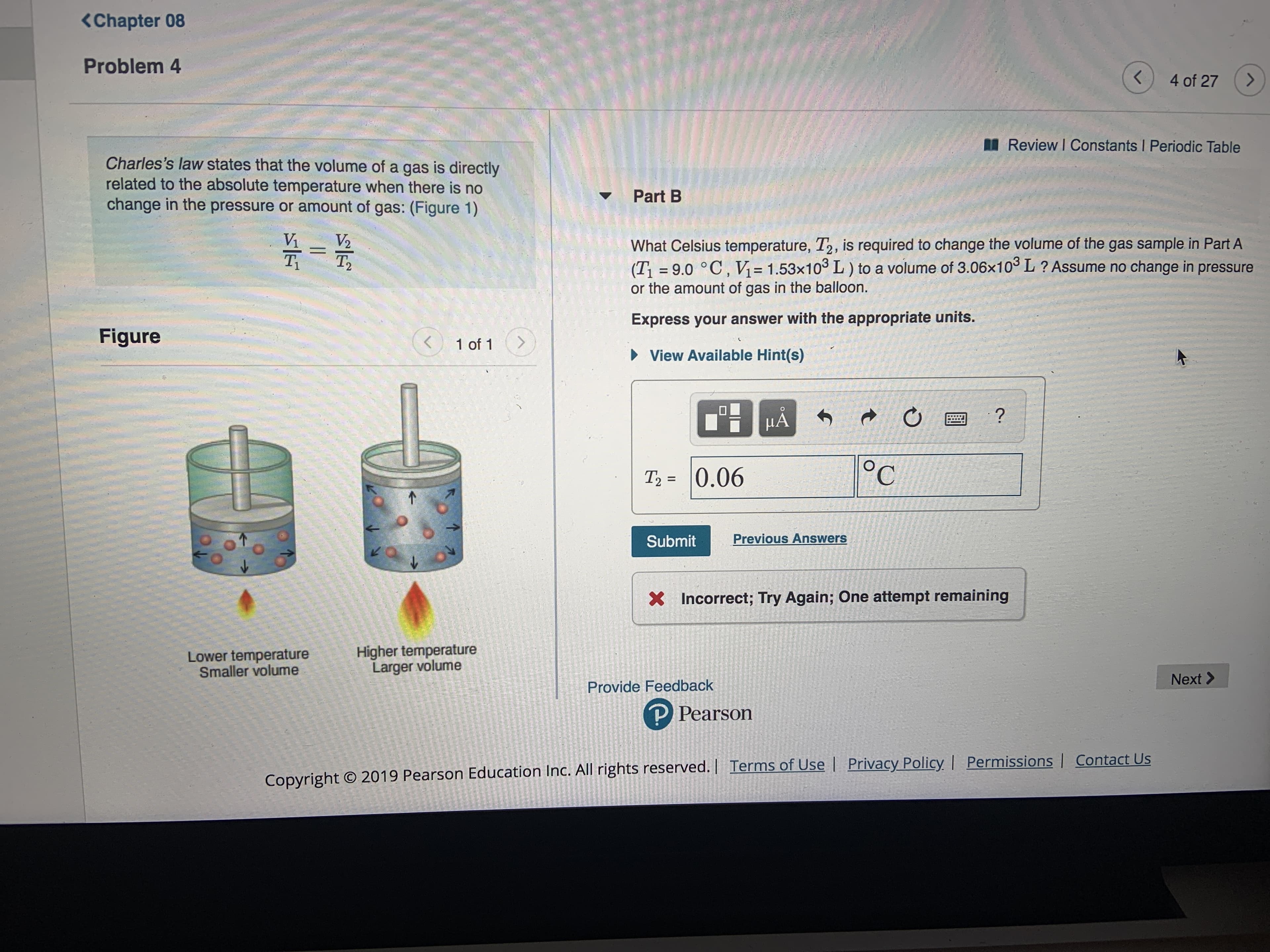
Transcribed Image Text:Chapter 08
Problem 4
r
4 of 27
Review I Constants I Periodic Table
Charles's law states that the volume of a gas
related to the absolute temperature when there is no
change in the pressure or amount of gas: (Figure 1)
is directly
Part B
Vi
V2
What Celsius temperature, T2, is required to change the volume of the gas sample in Part A
(T 9.0 ° C, V 1.53x103 L) to a volume of 3.06x10 L? Assume no change in pressure
or the amount of gas in the balloon.
T3
Ti
Express your answer with the appropriate units.
Figure
<
>
1 of 1
View Available Hint(s)
?
°C
T2= 0.06
Previous Answers
Submit
X
Incorrect; Try Again; One attempt remaining
Higher temperature
Larger volume
Lower temperature
Smaller volume
Next>
Provide Feedback
P Pearson
Copyright O 2019 Pearson Education Inc. All rights reserved.| Terms of Use | Privacy Policy | Permissions | Contact Us
का
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning