CHAPTER 7 - GASES, LIQUIDS, AND SOLIDS O Previous Page 14 of 36 Next O A mass of 1.36 g of an unknown gas exerts a pressure of 0.790 atm at 71°C in a 1.00-L flask. What is the molar mass of the gas? (R =0.08206 L-atm/mol-K O Previous Page 13 of 36 Next O What is the pressure exerted by 1.39 g Xe gas at 17.0 'C in a 340-mL flask?
CHAPTER 7 - GASES, LIQUIDS, AND SOLIDS O Previous Page 14 of 36 Next O A mass of 1.36 g of an unknown gas exerts a pressure of 0.790 atm at 71°C in a 1.00-L flask. What is the molar mass of the gas? (R =0.08206 L-atm/mol-K O Previous Page 13 of 36 Next O What is the pressure exerted by 1.39 g Xe gas at 17.0 'C in a 340-mL flask?
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter13: Fundamental Equilibrium Concepts
Section: Chapter Questions
Problem 61E: Complete the changes in concentrations (or pressure, if requested) for each of the following...
Related questions
Question
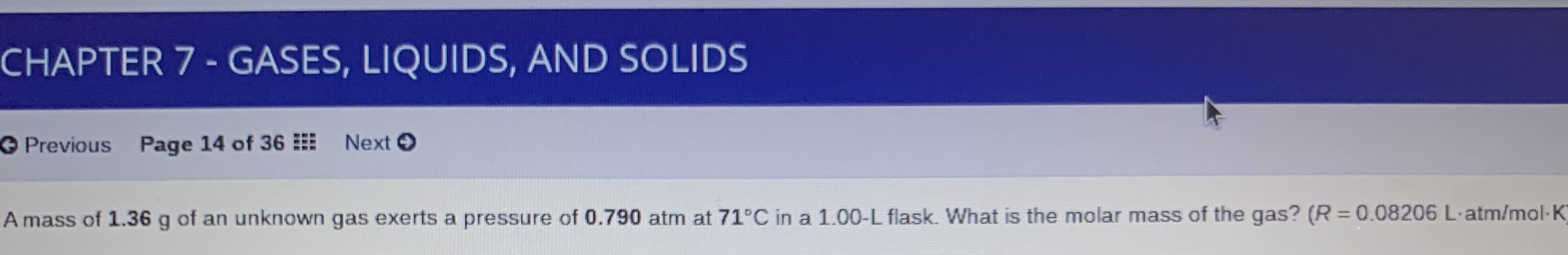
Transcribed Image Text:CHAPTER 7 - GASES, LIQUIDS, AND SOLIDS
O Previous Page 14 of 36
Next O
A mass of 1.36 g of an unknown gas exerts a pressure of 0.790 atm at 71°C in a 1.00-L flask. What is the molar mass of the gas? (R =0.08206 L-atm/mol-K
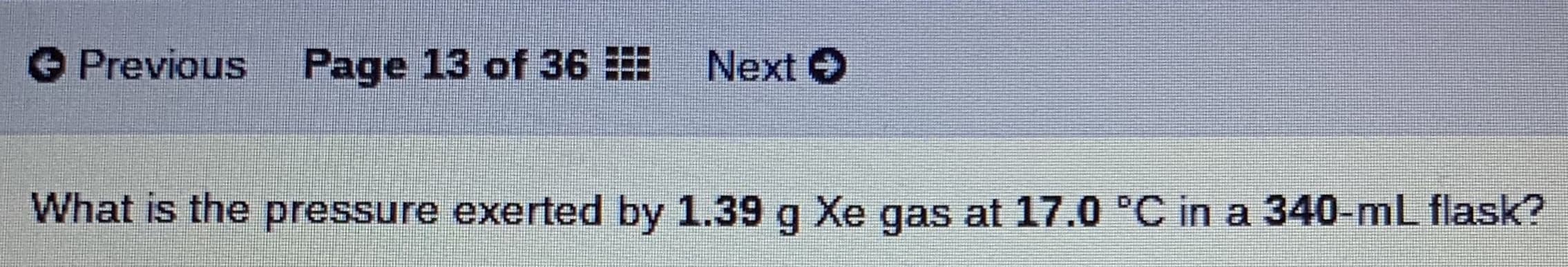
Transcribed Image Text:O Previous
Page 13 of 36
Next O
What is the pressure exerted by 1.39 g Xe gas at 17.0 'C in a 340-mL flask?
Expert Solution
The pressure has to be calculated.

Step 2
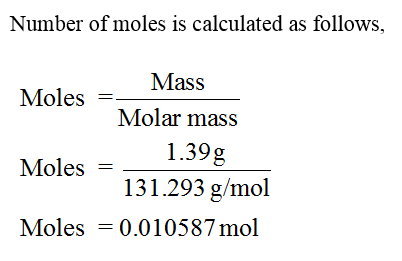
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
