Charcoal is primarily carbon. What mass of CO2 is produced if you burn enough carbon (in the form of charcoal) to produce 4.90 kJ × 10² kJ of heat? The balanced chemical equation is as follows: C(s) + O2 (g) → CO2 (g), ΔΗ -393.5 kJ Express the mass in grams to three significant figures. ο ΑΣφ т 3
Charcoal is primarily carbon. What mass of CO2 is produced if you burn enough carbon (in the form of charcoal) to produce 4.90 kJ × 10² kJ of heat? The balanced chemical equation is as follows: C(s) + O2 (g) → CO2 (g), ΔΗ -393.5 kJ Express the mass in grams to three significant figures. ο ΑΣφ т 3
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 90GQ: Water gas, a mixture of carbon monoxide and hydrogen, is produced by treating carbon (in the form of...
Related questions
Question
100%
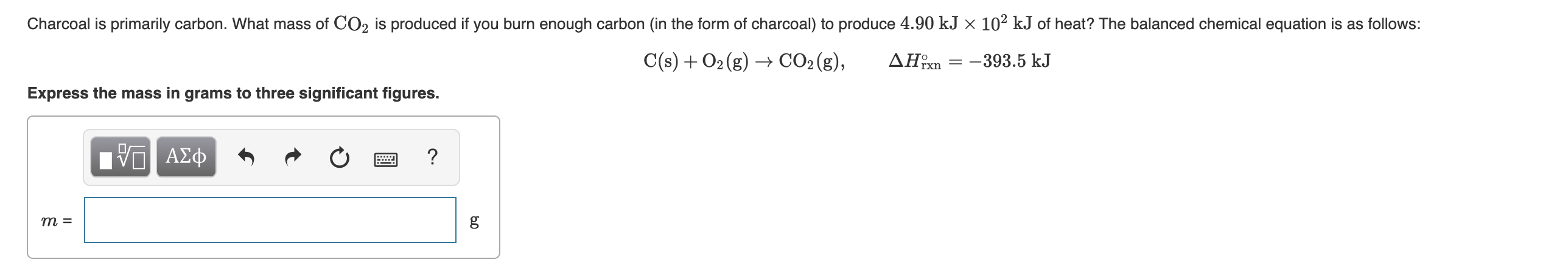
Transcribed Image Text:Charcoal is primarily carbon. What mass of CO2 is produced if you burn enough carbon (in the form of charcoal) to produce 4.90 kJ × 10² kJ of heat? The balanced chemical equation is as follows:
C(s) + O2 (g) → CO2 (g),
ΔΗ
-393.5 kJ
Express the mass in grams to three significant figures.
ο ΑΣφ
т 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
