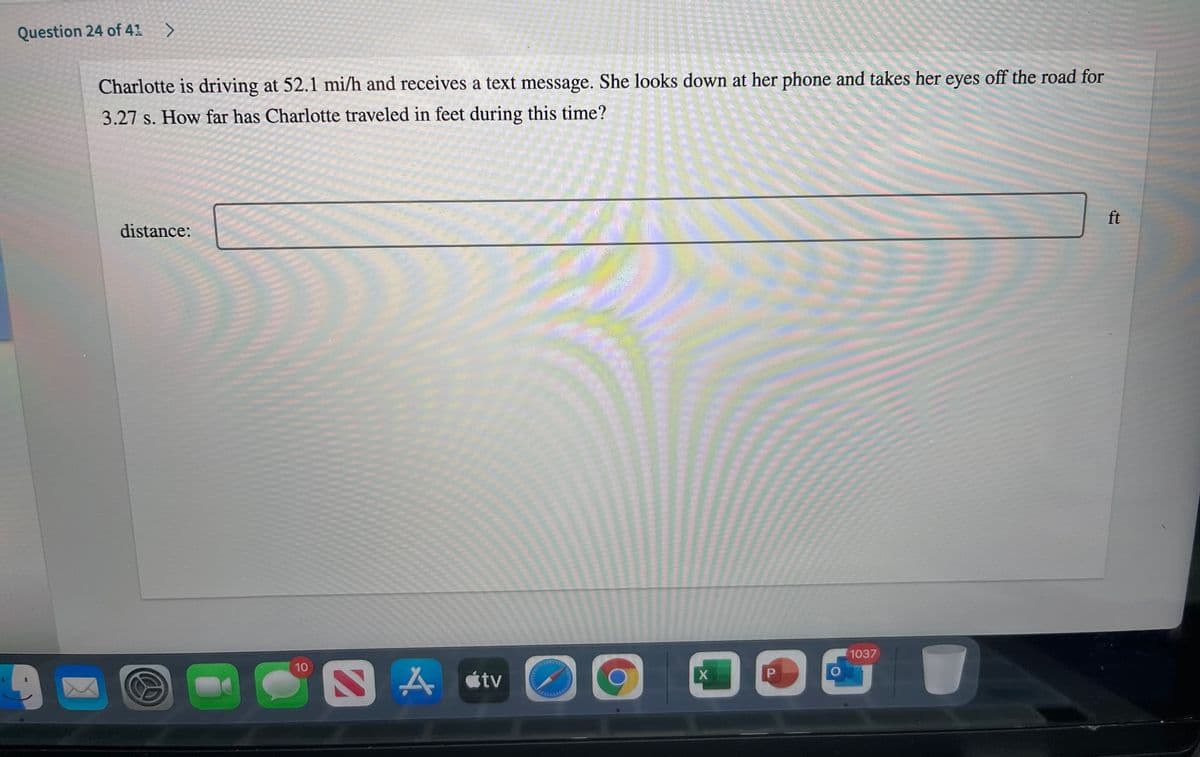
Charlotte is driving at 52.1 mi/h and receives a text message. She looks down at her phone and takes her eyes off the road for 3.27 s. How far has Charlotte traveled in feet during this time?
Charlotte is driving at 52.1 mi/h and receives a text message. She looks down at her phone and takes her eyes off the road for 3.27 s. How far has Charlotte traveled in feet during this time?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section: Chapter Questions
Problem 43SCQ: Define the terms renewable and nonrenewable as applied to energy resources. Which of the following...
Related questions
Question
Transcribed Image Text:Question 24 of 41 >
Charlotte is driving at 52.1 mi/h and receives a text message. She looks down at her phone and takes her eyes off the road for
3.27 s. How far has Charlotte traveled in feet during this time?
ft
distance:
1037
10
A átv
Transcribed Image Text:n 17 of 41 >
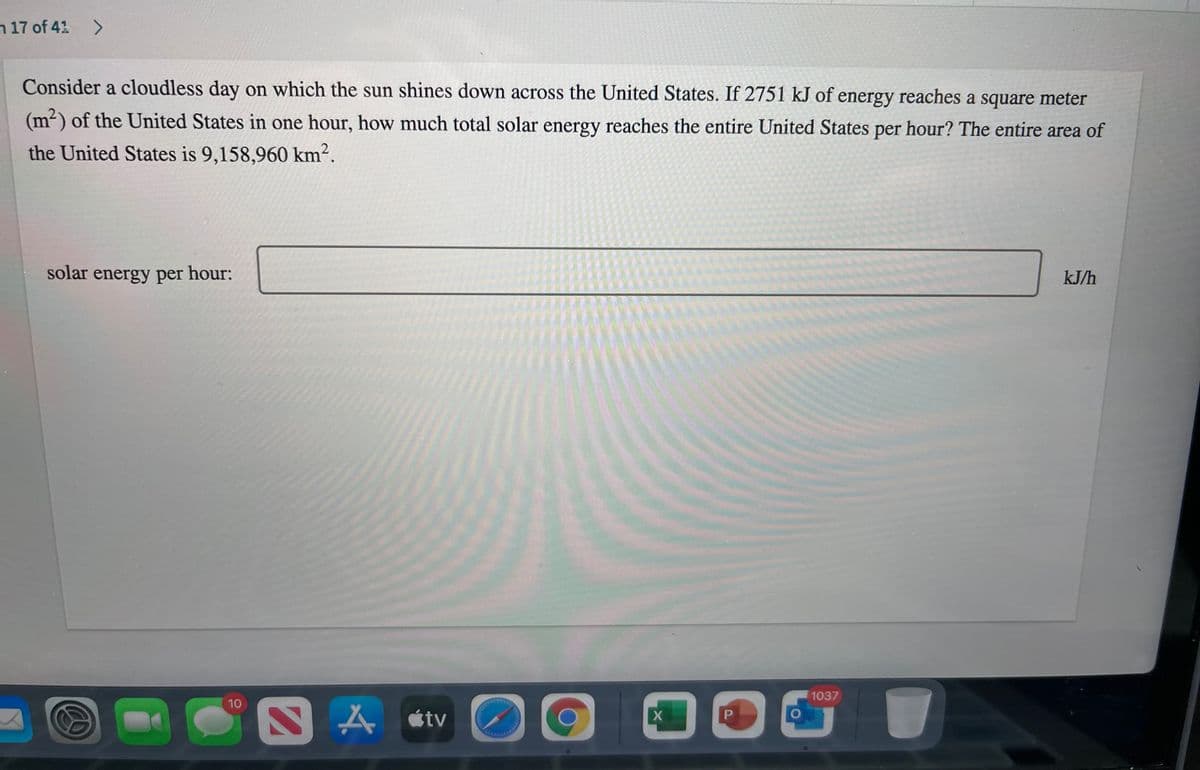
Consider a cloudless day on which the sun shines down across the United States. If 2751 kJ of energy reaches a square meter
(m²) of the United States in one hour, how much total solar energy reaches the entire United States per hour? The entire area of
the United States is 9,158,960 km².
solar energy per hour:
kJ/h
1037
10
A átv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning