< (O %#3 Two bulbs are connected by a stopcock. The 9.00 L bulb contains nitric oxide (NO) at a pressure of 0.517 bar, and the 3.00 L bulb contains oxygen (O,) at a pressure of 0.775 bar. After the stopcock is opened, the gases mix and react to produce nitrogen dioxide ON 0, (CON) (3) ON7- (3)°0+ (3)ON 7 Considering that the volume remains unchanged during the experiment, how does the total pressure in the bulbs change if the reaction is allowed to go to completion? O The total pressure will increase. O The total pressure will remain constant. There is not enough information to determine how the total pressure will change. The total pressure will decrease. MacBook Pro > delete 4. 8. 5. 6. 7. R. P. E. enter H. return B alt MOSISO command option
< (O %#3 Two bulbs are connected by a stopcock. The 9.00 L bulb contains nitric oxide (NO) at a pressure of 0.517 bar, and the 3.00 L bulb contains oxygen (O,) at a pressure of 0.775 bar. After the stopcock is opened, the gases mix and react to produce nitrogen dioxide ON 0, (CON) (3) ON7- (3)°0+ (3)ON 7 Considering that the volume remains unchanged during the experiment, how does the total pressure in the bulbs change if the reaction is allowed to go to completion? O The total pressure will increase. O The total pressure will remain constant. There is not enough information to determine how the total pressure will change. The total pressure will decrease. MacBook Pro > delete 4. 8. 5. 6. 7. R. P. E. enter H. return B alt MOSISO command option
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 155CP: Methane (CH4) gas flows into a combustion chamber at a rate of 200. L/min at 1.50 atm and ambient...
Related questions
Question
Transcribed Image Text:< (O
%#3
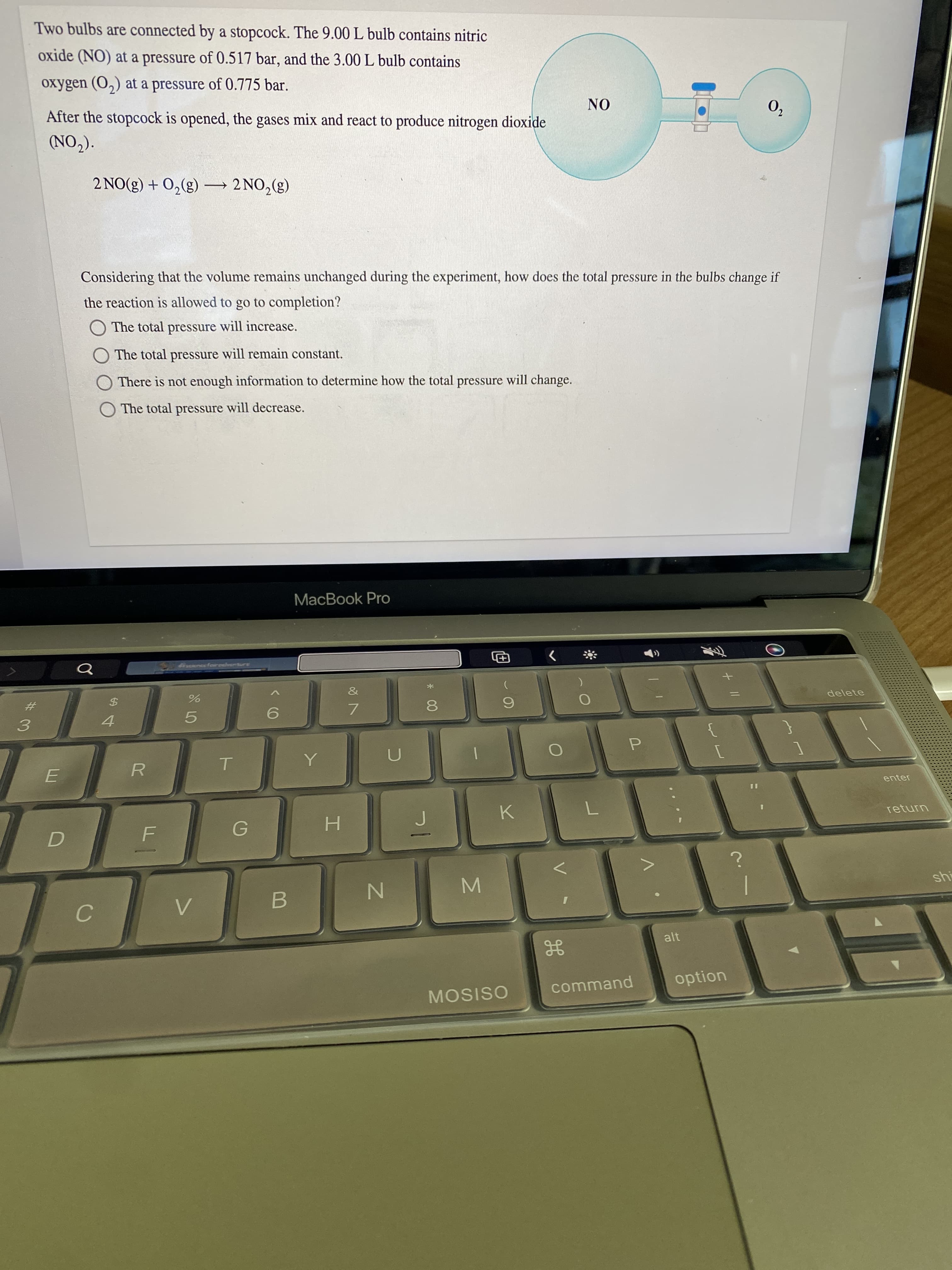
Two bulbs are connected by a stopcock. The 9.00 L bulb contains nitric
oxide (NO) at a pressure of 0.517 bar, and the 3.00 L bulb contains
oxygen (O,) at a pressure of 0.775 bar.
After the stopcock is opened, the gases mix and react to produce nitrogen dioxide
ON
0,
(CON)
(3) ON7- (3)°0+ (3)ON 7
Considering that the volume remains unchanged during the experiment, how does the total pressure in the bulbs change if
the reaction is allowed to go to completion?
O The total pressure will increase.
O The total pressure will remain constant.
There is not enough information to determine how the total pressure will change.
The total pressure will decrease.
MacBook Pro
>
delete
4.
8.
5.
6.
7.
R.
P.
E.
enter
H.
return
B
alt
MOSISO
command
option
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning