Q: Calculate the Ecel of the following reaction Zno + Ni*²(aq) Zn*2(aq) Ni(s) When Ni*2 = 0.85 M and…
A: [Ni+2] = 0.85 M [Zn+2] = 0.45 M We need to find emf of given cell reaction.
Q: What is ΔH°rxn for the following reaction? C6H6(l) + 15/2 O2(g) → 6CO2(g) + 3H2O(l) 6C(graphite)…
A: ΔH°rxn for the reaction is to be calculated
Q: A GO0 L vessel phosphorus pentach loride and en equil'brium tHhe reactn 3.1. Contained O,0 222 mol…
A: Given data : 1) Moles of phosphorus trichloride = 0.0222 mol Moles of phosphorus pentachloride =…
Q: Given the reaction P,O10 (s) + H2O → H3 PO4 (aq) ΔΗ -96 %3D .2 kJ what the AH for P4O10 (s) + 6 H2O…
A:
Q: 2) Calculate AH for the reaction below using Hess' law. Light and the ctron AH=? C¿H6(g) → C2H2(g) +…
A: Hess law states that the enthalpy change during the reaction remains same even if followed different…
Q: Be sure to answer all parts. Use the table to calculate ΔG for the following reactions at 25°C.…
A: (a)ΔG for the given reaction at 25°C is calculated as follows,
Q: balancing equations C2H6+O2=CH3COOH
A: Given equation is:- C2H6+O2=CH3COOH+H2O
Q: 4. Using the AHº values from the table below, calculate AH°xn for the following reaction. 4 NH3 (g)…
A: We have to calculate the ∆Ho for reaction.
Q: Enter your answer in the provided box. Find AG for the following reaction, using AH and S values.…
A: Given reaction is H2 (g) + I2 (s) → 2 HI (g) Standard thermodynamics data are (at T=298 K)…
Q: Which of the following processes have a AS > 0? O CH3OH(1) – CH30H(s) O N2(g) + 3 H2(g) – 2 NH3(g) O…
A:
Q: How do I solve this
A:
Q: In terms of % yield = (actual / theorietical ) X 100, explain what is meant by “theorietical”.
A: Answer :- Theoretical yield :- In a chemical reaction Theoretical yield is the maximum quantity of a…
Q: What is ASuniv at 25 °C for the reaction 2 S(s) + Cl2(g) - S2Cl2(g)?
A: 1) The reaction taking place is given as,
Q: Use thermochemical data to compute AH° for the reaction C(s) + O2(g) → CO2(g) at 1000°C, the…
A: This problem can be solved using Kirchhoff's equation: ∆H(1000C0)=∆H(25C0)+Cp∆T
Q: Urea, CO(NH2)2 is slowly converted to ammonia (NH3) at 25.0 °C according to the following reaction.…
A: The reaction for decomposition of urea is,CO(NH2)2 (aq) + H2O (l) → CO2 (aq) + NH3 (aq)Heat of the…
Q: Calculate the value of AH° for the reaction. 2 H2S (g) + 3 02 (g) > 2 H2O (1) + 2 SO2 (g) AH°F H2S…
A: The standard enthalpy of formation is the enthalpy change of reaction in standard conditions when…
Q: Consider the reaction A B with AH° = -42.46 kJ/molxn- What should be the AH° in kJ/moln for the…
A: Consider the given target equation is as follows; 3 B → 3 A ∆H°=?…
Q: Calculate the AGᵒrxn using the following information at 25 °C 7 N₂(g) + H₂O(1) HNO3(g) + N₂H4(1)…
A:
Q: Determine the ΔS0rxn of the reaction below: N2H4(l) + N2O4(g) → N2(g) + H2O(g) ΔS0f (J/K)…
A: Ans
Q: J cal kJ 688 199 0.585
A: 1 cal = 4.184 J 1kJ = 1000 J
Q: 19) What is AH°rxn for the following reaction? C6H6(1) + 15/2 O2(g)→6CO2(g) + 3H2O(1) 6C(graphite) +…
A: Given reaction is C6H6 (l) + 15/2 O2 (g) → 6 CO2 (g) + 3 H2O(l) ------- (1) 6…
Q: Given: 2 Ag+(aq) + Cu(s) ⟶ 2 Ag(s) + Cu2+(aq) ΔGo = -88.66 kJ/mol Calculate the…
A: The given balanced redox Chemical Reaction is - 2 Ag+(aq) + Cu(s) ⟶ 2 Ag(s) + Cu2+(aq)…
Q: Calculate ΔG° using the following reaction and information below . CH3CH2OH(g) + 3O2(g) ---> 2CO2(g)…
A: The relation between ΔG°, ΔH°, and ΔS° is as follows ΔG°=ΔH° – TΔS° Where T is temperature and…
Q: 1. You have the following reaction at 25°C Sg(s) + 24F2(g) 8SF,(g) a. Calculate AG- The AGarmation…
A: Given: The reaction is as follows: S8g+24F2g→8SF6g ∆Gformation=-1296 KJ/mol. ∆Hformation=-1209…
Q: Calculate the value of AH for the reaction: 3 Fe (s) + 4 H20 (g) Fe304 (s) + 4 H2 (g) Fe (s) + H20…
A:
Q: 06) Responda às questões a seguir para cada uma das substâncias: a) Quantos sinais aparecem no…
A:
Q: Consider the problem below: (Equation 1) H2(g) + 1/2 O2(g) ---> H2OU) AH = -285.8 kJ/mol (Equation…
A: The enthalpy change is a state function and the Hess law states that the change of enthalpy in a…
Q: Calculate (triangle)G for: C2H4 (g) + H2O (g) -> C2H5OH(g)
A: Thermodynamic is the branch of chemistry that mainly deals with the heat transfer between system and…
Q: Consider the following reaction: C3H18(1) + 25/2 O»(g) → 8 CO2(g) + 9 H20(1) If d[CO2l/dt =…
A:
Q: Calculate the heat of reaction of each reaction: (assume density of solution is 1.04 g/ml and…
A:
Q: Calculate AG°xn for the following reaction of white phosphorous with HCl. 2P (s) + 10HC1 (g) → 2PCI5…
A: The balanced reaction taking place is given as, => 2 P (s) + 10 HCl (g) -------> 2 PCl5 (g) +…
Q: 1. What is the mass percent of potassium chloride in a solution that is made by dissolving 15.0 g…
A: Given:Mass of KCl = 15.0 gMass of H2O = 100.0 g
Q: Which of the following processes has ΔS<0? Select one: C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) 1.0 L of…
A: The study of flow of matter is called thermodynamics. Entropy is an important term used in…
Q: Calculate ∆Horxn for the following reaction 2H2O2(l) 2H2O(l) + O2(g) given that…
A:
Q: 2. You are studying the hydration of compound X, which is represented by the reaction X + H2O=X –…
A: Given that
Q: AH, = -393.5 kJ AH, = -283.0 kJ (1) C(s) + O2(8) CO2(g) (2) Cog) + 028) – CO2(8) (3) C(s) + 0:8)…
A: C(s) + O2(g) ——> CO2 (g) …….1) CO(g) + 1/2 O2(g) ——> CO2 (g) ……..2) subtract equation 2)…
Q: 14. Calculate the Ke of the reaction 2NO (g) + Br2 (g) following information: 2NOBR (g) from the…
A: Given,
Q: 5. NaHCC b. Ca(HCO;), с. KHC,0,
A: Name of the given compound and if possible used 'bi' ---
Q: Use the following values of standard entropies and ΔSrxn to determine the missing standard…
A:
Q: Given the following reactions A (g) +B (g) – 2 C (g) AH = +810.7 kJ 2 C (g) +B (g) → 2 D (g) AH =-…
A: To calculate the enthalpy of desired reaction , we have to use the given reactions. We have to…
Q: 3- For the reaction H2(g) + I2(g)2 2HI(g) at 425°C, calculate [HI], given [H2] = [I2] = 4.79 × 10ª…
A: “Since you have asked multiple question, we will solve the first question for you. If you want any…
Q: i If concentration of [CrO4 ] in Expt. 7 is found to be 6.7 x 10 M, what is the experimental Ksp of…
A:
Q: This questin has mstiple parts. Wark al the parts te pet the most OFor the following set of…
A: According to Charles's law, at constant pressure : V1T1=V2T2Here V1 and V2 are initial and final…
Q: I need to use sodium phosphate monobasic to make a sodium phosphate solution of .01 milimol/l but…
A: Sodium phosphate monobasic or mono sodium phosphate is NaH2PO4.
Q: Calculate the AHxn/mol Ca for the reaction: Ca (s) + 2 HCl(aq)-CaCl₂(aq) + H₂(g) when 0.400 g Ca…
A: Here we are required to find the Enthalpy for the reaction. Given Specific heat capacity C =4.15 J…
Q: For the following reaction, indicate the false statement. T/I H₂O + CI HCI + H₂O
A:
Q: Given the following data 2 CIF(9) + O2 (g) Cl2 O(g)+ F20(g) 2 CIF3 (g) + 202(9) → Cl2 O(g) + 3F,0(g)…
A:
Q: Species AG° Fe(OH)3 -696.5 3+ -4.6 OH -157.3 Calculate the AG° of the reaction: Fe(OH)3(s) = Fe*(aq)…
A: Given values- ∆G° of Fe(OH)3=-696.5 ∆G° of Fe^3+=-4.6 ∆G° of OH^-=-157.3
Q: P4.41 The lactic acid byproduct recovered from cheese plants can be used to make a variety of…
A: The explanation is given below-
Q: If Q = 71.3 and Keg = 0.227, the reaction favors Products (A) (B) Reactants
A: If, Qc < Kc, the ratio of initial concentrations of products to reactants is too small. To get a…
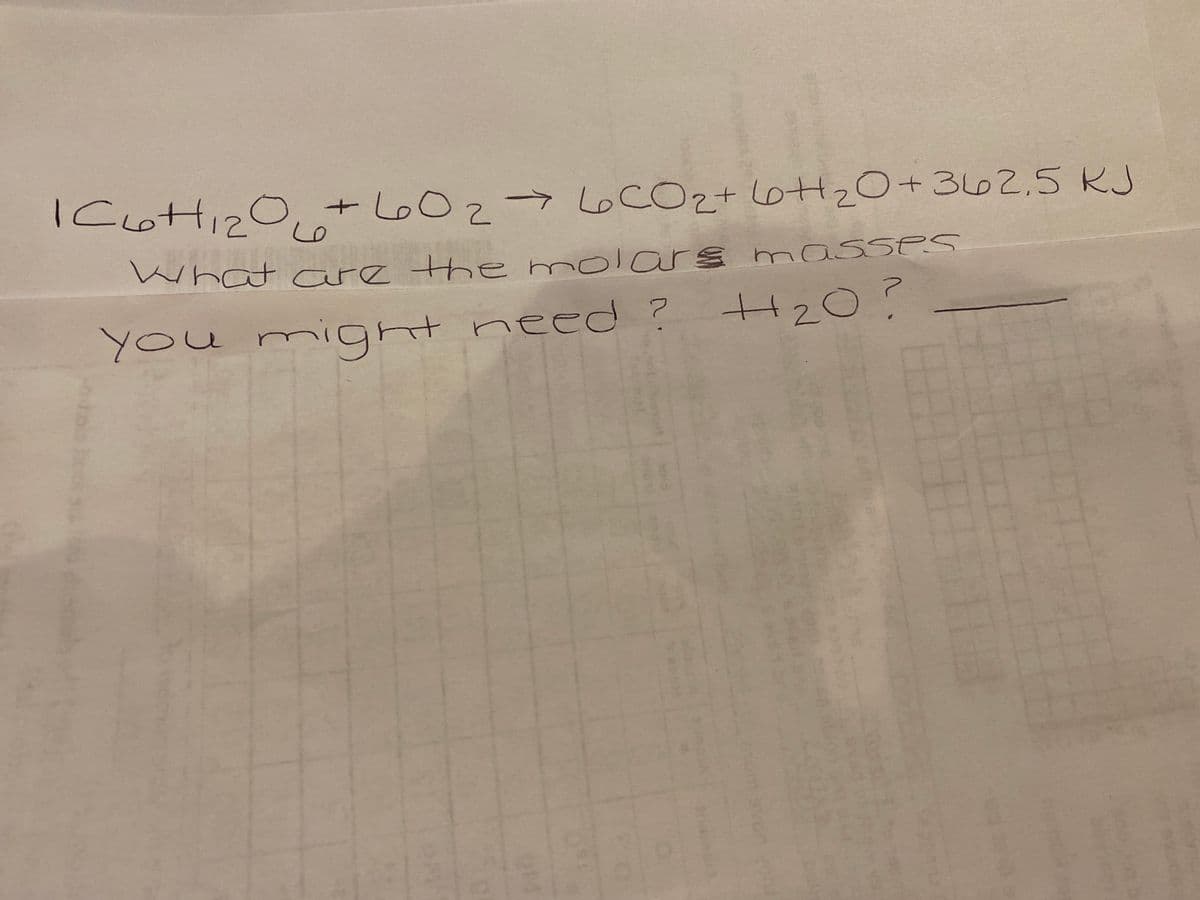
Step by step
Solved in 2 steps

- Calculate ΔG^0cell in kJ/mol at 621 K givenE^0cell= -0.0700 voltsn= 5F= 96500 C/mole electronsΔG^0cell=−nFE^0cell answer choices: a. 0.000350 b. 15.0 c. 6.76 d. 33.8 e. 112What is the value of Ered when log3Ag+4 = 0?calculate delta H for the following unbalanced reaction: C6H12O6 + O2 (g) --> CO2 (g) + H2O (g)
- Calculate the ΔH for the reaction:CS2 (l) + 2 O2(g) CO2 (g) + 2 SO2 (g)Given:ΔHfCO2 (g) = - 393.5 kJ/mol;ΔHfSO2 = -296.8 kJ/mol;ΔHfCS2 (l) = 87.9kJ/molCalculate the ΔH for the reaction: CS2 (l) + 2 O2(g) CO2 (g) + 2 SO2 (g) Given: ΔHfCO2 (g) = - 393.5 kJ/mol; ΔHfSO2 = -296.8 kJ/mol; ΔHfCS2 (l) = 87.9kJ/molCalculate ∆Horxn for the following reaction 2H2O2(l) 2H2O(l) + O2(g)given that ∆Hof [H2O(l)] = -285.8 kJ/mol and ∆Hof [H2O2(l)] = -187.6 kJ/mol.
- iven the data in the table below, ΔH°rxn for the reaction C2H5OH (l) + O2 (g) → CH3CO2H (l) + H2O (l) is ________ kJ. -79.0 -1048.0 -476.4 -492.6 The value of ΔH°f of O2 (g) is required for the calculation.When ln(Ksp) is plotted vs 1/T(K), the graph has a y-intercept of 44.17. Calculate dS in units of J/K.mol and report only the numerical answer in the box below.Calculate qtotal for 2.0 mol of I2 (M = 253.8 g mol-1; theta r = 0.054 K; theta v =310 K; g0 = 1; De = 35.9 kcal mol-1) at 300 K and 2.0 atm pressure. Assumeideal behavior (except in the calculation of qvib).
- Calculate ΔG0 (in kJ/mol) given ΔG= -423.1 kJ/mol and R= 0.008314 kJ/mol K and T= 611.3 K and Q=0.601ΔG=ΔG0+RTlnQWhich of the following is a spontaneous reaction.? a. Rxn with ΔH =- 10Kj/mol ΔS= -5J/mol T= 300K b. NaCl +H20 -> NaOH + HCl 25C c. H20(l) -> H2O(s) Temp: 25C d. Dissolution of 100g of solid sugar in 100 mL ice tea. Consider following reaction: HgO (s) -> Hg(l) + ½ O2 (g) Delta H = +90.7 kj/mol. What quantity of heat in kj/mol is required to produce one mole HgO? Write your answer without units. Given the following data 2ClF(g) + O2(g) --> Cl2O(g) + F2O (g) Delta H= 167.4 kJ I 2ClF3(g) + 2O2(g) --> Cl2O(g) + 3F2 O (g) Delta H= 341.4 kJ II 2 F2(g) + O2(g) ---> 2F2O (g) Delta H= -43.4 kJ III Calculate the delta H in kJ for below reaction: ClF(g) + F2(g) ---> ClF3(g)What would be the equilibrium temperature if you drop 5.0 g of brass (Cp= 0.38 J/g-°C, initially at 68°C) and 3.5 g of aluminum (Cp = 0.9 J/g-°Cinitially at 83°C) into 105 g of water (Cp = 4.184 J/g-°C,initially at 23°C)?



