Limestone Oyster Shell 94.1430g 97.2738 Mass of flask + acid 14901a 1.5000g 0.7025g Mass of boat + sample 0-11.04 97.7069g Mass of boat Mass of flask + acid + sample at 20 min 94.62049 Reactions Limestone CaCO,(s) + MgCO,(s) + 4 HCl(aq) → CaCl,(aq) + MgCl,(aq) + 2 CO,(g) + H,O(1I) CACO,(s) + 2 HCI(aq) → CaCl,(aq) + CO,(g) + H,0(1) Oyster Shell Calculated Values Limestone Oyster Shell 0-7697g 9804354 Mass of sample 0.7975g 94.9405g 0-3201g Initial mass of flask + acid + sample 0.336 Mass of CO, lost at 20 min Moles of CO2 0.0072734 mol 0.0076483mal 8.0072734 mol 6.0076483md Moles of CO, 2- 3 0,0036367 mol 0.0076483 mal Moles of CACO, Moles of MgCO, 0.0036367md Total mass of MGCO, + CACO, Mass percent (MgCO, + CaCO,) 3. Mass of CaCO, 3. Mass percent CaCO, 3.
Limestone Oyster Shell 94.1430g 97.2738 Mass of flask + acid 14901a 1.5000g 0.7025g Mass of boat + sample 0-11.04 97.7069g Mass of boat Mass of flask + acid + sample at 20 min 94.62049 Reactions Limestone CaCO,(s) + MgCO,(s) + 4 HCl(aq) → CaCl,(aq) + MgCl,(aq) + 2 CO,(g) + H,O(1I) CACO,(s) + 2 HCI(aq) → CaCl,(aq) + CO,(g) + H,0(1) Oyster Shell Calculated Values Limestone Oyster Shell 0-7697g 9804354 Mass of sample 0.7975g 94.9405g 0-3201g Initial mass of flask + acid + sample 0.336 Mass of CO, lost at 20 min Moles of CO2 0.0072734 mol 0.0076483mal 8.0072734 mol 6.0076483md Moles of CO, 2- 3 0,0036367 mol 0.0076483 mal Moles of CACO, Moles of MgCO, 0.0036367md Total mass of MGCO, + CACO, Mass percent (MgCO, + CaCO,) 3. Mass of CaCO, 3. Mass percent CaCO, 3.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.22QAP
Related questions
Question
I need help with the last four calculated values please!!!!
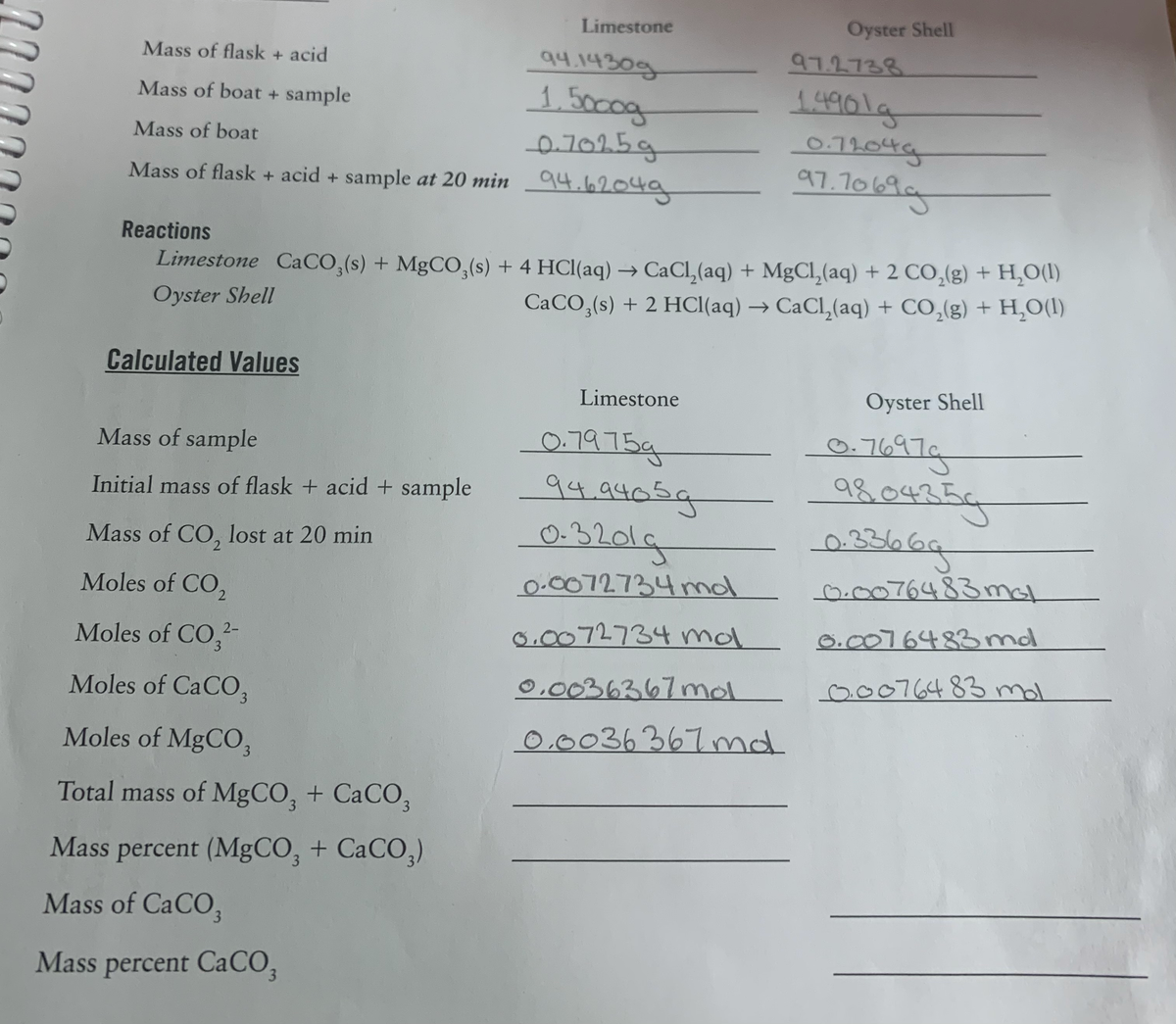
Transcribed Image Text:Limestone
Oyster Shell
94.1430g
97.2738
Mass of flask + acid
14901a
1.5000g
0.7025g
Mass of boat + sample
0-11.04
97.7069g
Mass of boat
Mass of flask + acid + sample at 20 min 94.62049
Reactions
Limestone CaCO,(s) + MgCO,(s) + 4 HCl(aq) → CaCl,(aq) + MgCl,(aq) + 2 CO,(g) + H,O(1I)
CACO,(s) + 2 HCI(aq) → CaCl,(aq) + CO,(g) + H,0(1)
Oyster Shell
Calculated Values
Limestone
Oyster Shell
0-7697g
9804354
Mass of sample
0.7975g
94.9405g
0-3201g
Initial mass of flask + acid + sample
0.336
Mass of CO, lost at 20 min
Moles of CO2
0.0072734 mol
0.0076483mal
8.0072734 mol
6.0076483md
Moles of CO,
2-
3
0,0036367 mol
0.0076483 mal
Moles of CACO,
Moles of MgCO,
0.0036367md
Total mass of MGCO, + CACO,
Mass percent (MgCO, + CaCO,)
3.
Mass of CaCO,
3.
Mass percent CaCO,
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
