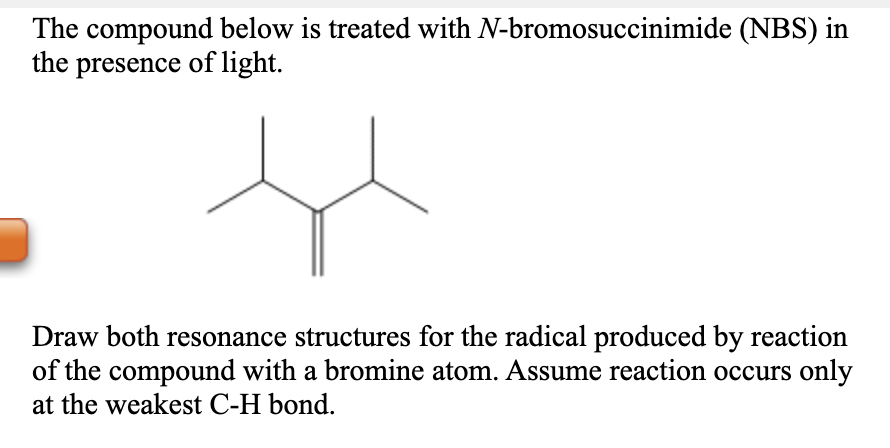
The compound below is treated with N-bromosuccinimide (NBS) in the presence of light. Draw both resonance structures for the radical produced by reaction of the compound with a bromine atom. Assume reaction occurs only at the weakest C-H bond.
Electronic Effects
The effect of electrons that are located in the chemical bonds within the atoms of the molecule is termed an electronic effect. The electronic effect is also explained as the effect through which the reactivity of the compound in one portion is controlled by the electron repulsion or attraction producing in another portion of the molecule.
Drawing Resonance Forms
In organic chemistry, resonance may be a mental exercise that illustrates the delocalization of electrons inside molecules within the valence bond theory of octet bonding. It entails creating several Lewis structures that, when combined, reflect the molecule's entire electronic structure. One Lewis diagram cannot explain the bonding (lone pair, double bond, octet) elaborately. A hybrid describes a combination of possible resonance structures that represents the entire delocalization of electrons within the molecule.
Using Molecular Structure To Predict Equilibrium
Equilibrium does not always imply an equal presence of reactants and products. This signifies that the reaction reaches a point when reactant and product quantities remain constant as the rate of forward and backward reaction is the same. Molecular structures of various compounds can help in predicting equilibrium.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images


