Consider the reaction: NH2COONH4 (s) = 2 NH3 (g) + CO2 (g) At high temperatures such as 250.°C, the equilibrium constant for the reaction is 1.58 x 10-8. A research student ran the experiment by first placing 39.1 g of the reactant compound in a 750. mL evacuated closed container. (R = 0.08206 L.atm/K.mol; K = °C + 273; pay attention to sig figs) a) Write the equilibrium expression, Kc, for this reaction. kc=l.58 x 10 COONH kc [NH3]? [COz] b) What is the concentration of each of the products at equilibrium? NH2COONH4 (s) = 2 NH3 (g) + CO2 (g) NHz COON H4: 39.1g 78.070 gimol 50 mol NH2 COON Hy = Z NH3 CO2 Tnitial -50 gimo1 W 999. + 2 X t ズ ミGGG N •150L E : 666-X answer: ENHS] = 2(-89x104) :1.78x10-4 M C COz] = -89x 10 4 M kc CNH3]?CCO1] →1.58* 10°8-(2x) Cx) 2x2.1.58x 1 X: 1.58x108 c) What is the pressure in atm exerted by each of the products at equilibrium? on 79x 10 こ、 kp= kc x (RT) X= .89x104 R=. 08206 kp:1.58x10-8 x(.08206x523)? :1,58 x 108x (42.91738)? : 2.9|x 10-5 n (3-1) = 2 入 T 523 k -5 [NH3]=2(,0302) = . O605 altm X= .0302 COL): 10302 atm d) Use these pressures to calculate the Kp for the reaction at this temperature. d) Use these pressures to calculate the K, for the reaction at this temperature. e) What is the total pressure in the container at equilibrium. f) If the reaction was initially set up with 95.5 g of the reactant instead of the 39.1 g, and all other conditions were the same , what are the concentrations of each of the products at equilibrium?
Consider the reaction: NH2COONH4 (s) = 2 NH3 (g) + CO2 (g) At high temperatures such as 250.°C, the equilibrium constant for the reaction is 1.58 x 10-8. A research student ran the experiment by first placing 39.1 g of the reactant compound in a 750. mL evacuated closed container. (R = 0.08206 L.atm/K.mol; K = °C + 273; pay attention to sig figs) a) Write the equilibrium expression, Kc, for this reaction. kc=l.58 x 10 COONH kc [NH3]? [COz] b) What is the concentration of each of the products at equilibrium? NH2COONH4 (s) = 2 NH3 (g) + CO2 (g) NHz COON H4: 39.1g 78.070 gimol 50 mol NH2 COON Hy = Z NH3 CO2 Tnitial -50 gimo1 W 999. + 2 X t ズ ミGGG N •150L E : 666-X answer: ENHS] = 2(-89x104) :1.78x10-4 M C COz] = -89x 10 4 M kc CNH3]?CCO1] →1.58* 10°8-(2x) Cx) 2x2.1.58x 1 X: 1.58x108 c) What is the pressure in atm exerted by each of the products at equilibrium? on 79x 10 こ、 kp= kc x (RT) X= .89x104 R=. 08206 kp:1.58x10-8 x(.08206x523)? :1,58 x 108x (42.91738)? : 2.9|x 10-5 n (3-1) = 2 入 T 523 k -5 [NH3]=2(,0302) = . O605 altm X= .0302 COL): 10302 atm d) Use these pressures to calculate the Kp for the reaction at this temperature. d) Use these pressures to calculate the K, for the reaction at this temperature. e) What is the total pressure in the container at equilibrium. f) If the reaction was initially set up with 95.5 g of the reactant instead of the 39.1 g, and all other conditions were the same , what are the concentrations of each of the products at equilibrium?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 61QRT
Related questions
Question
I need help with parts d, e, and f, please
![Consider the reaction:
NH2COONH4 (s) = 2 NH3 (g) + CO2 (g)
At high temperatures such as 250.°C, the equilibrium constant for the reaction is 1.58 x 10-8. A research
student ran the experiment by first placing 39.1 g of the reactant compound in a 750. mL evacuated closed
container. (R = 0.08206 L.atm/K.mol; K = °C + 273; pay attention to sig figs)
a) Write the equilibrium expression, Kc, for this reaction.
kc=l.58 x 10
COONH
kc [NH3]? [COz]
b) What is the concentration of each of the products at equilibrium?
NH2COONH4 (s) = 2 NH3 (g) + CO2 (g)
NHz COON H4: 39.1g
78.070 gimol
50 mol
NH2 COON Hy = Z NH3 CO2
Tnitial -50 gimo1
W 999.
+ 2 X
t ズ
ミGGG N
•150L
E
: 666-X
answer: ENHS] = 2(-89x104)
:1.78x10-4 M
C COz] = -89x 10 4 M
kc CNH3]?CCO1] →1.58* 10°8-(2x) Cx)
2x2.1.58x 1
X: 1.58x108
c) What is the pressure in atm exerted by each of the products at equilibrium?
on
79x 10
こ、
kp= kc x (RT)
X= .89x104
R=. 08206
kp:1.58x10-8 x(.08206x523)?
:1,58 x 108x (42.91738)?
: 2.9|x 10-5
n (3-1) = 2
入
T 523 k
-5
[NH3]=2(,0302) = . O605 altm
X= .0302
COL): 10302 atm
d) Use these pressures to calculate the Kp for the reaction at this temperature.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fc15907a8-70f3-4470-885a-42b8107f503f%2Fc746840e-5be2-449c-abca-459ee1b7b0e1%2F6y7oeji_processed.png&w=3840&q=75)
Transcribed Image Text:Consider the reaction:
NH2COONH4 (s) = 2 NH3 (g) + CO2 (g)
At high temperatures such as 250.°C, the equilibrium constant for the reaction is 1.58 x 10-8. A research
student ran the experiment by first placing 39.1 g of the reactant compound in a 750. mL evacuated closed
container. (R = 0.08206 L.atm/K.mol; K = °C + 273; pay attention to sig figs)
a) Write the equilibrium expression, Kc, for this reaction.
kc=l.58 x 10
COONH
kc [NH3]? [COz]
b) What is the concentration of each of the products at equilibrium?
NH2COONH4 (s) = 2 NH3 (g) + CO2 (g)
NHz COON H4: 39.1g
78.070 gimol
50 mol
NH2 COON Hy = Z NH3 CO2
Tnitial -50 gimo1
W 999.
+ 2 X
t ズ
ミGGG N
•150L
E
: 666-X
answer: ENHS] = 2(-89x104)
:1.78x10-4 M
C COz] = -89x 10 4 M
kc CNH3]?CCO1] →1.58* 10°8-(2x) Cx)
2x2.1.58x 1
X: 1.58x108
c) What is the pressure in atm exerted by each of the products at equilibrium?
on
79x 10
こ、
kp= kc x (RT)
X= .89x104
R=. 08206
kp:1.58x10-8 x(.08206x523)?
:1,58 x 108x (42.91738)?
: 2.9|x 10-5
n (3-1) = 2
入
T 523 k
-5
[NH3]=2(,0302) = . O605 altm
X= .0302
COL): 10302 atm
d) Use these pressures to calculate the Kp for the reaction at this temperature.
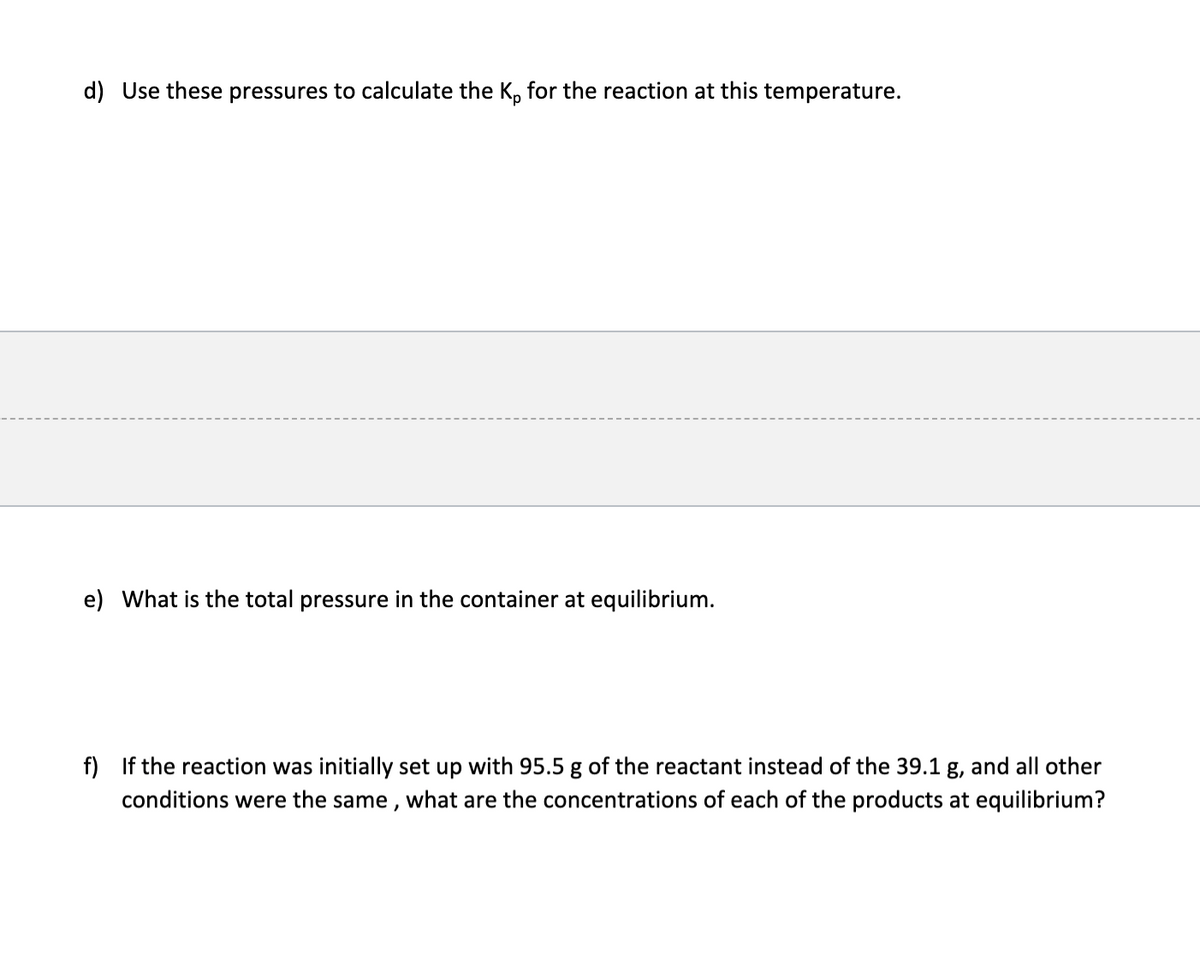
Transcribed Image Text:d) Use these pressures to calculate the K, for the reaction at this temperature.
e) What is the total pressure in the container at equilibrium.
f) If the reaction was initially set up with 95.5 g of the reactant instead of the 39.1 g, and all other
conditions were the same , what are the concentrations of each of the products at equilibrium?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning