8 leonline.hccs.edu/courses/188795/assignments/3601808 Homework Ch16 - Attempt 1 roblem 16.119 5 of 15 > Constants | Periodic Table Atmospheric CO2 levels have risen by nearly 20% over the past 40 years from 315 ppm to 380 ppm. What volume of CO2 at 25°C and 1.0 atm is dissolved in a 18.0-L bucket of today's rainwater? Express your answer using two significant figures. Vco, hp Leve Flectroaica Co, Lad ting Shicboog Privae lndustrial Acca, Tova Dongguaa ciy,Chins ADVA f12. fs insert 84 64 4 V. 5. 6. N Careers Mail - boss Texas Work HOc HomewcX W College Ph + eonline.hccs.edu/courses/188795/assignments/3601808 omework Ch16 - Attempt 1 oblem 16.119 <> 05 of 15 > Constants | Periodic Table Part A Atmospheric CO2 levels have risen by nearly 20% over the past 40 years from 315 ppm to 380 ppm. Given that the average pH of clean, unpolluted rain today is 5.4, determine the pH of unpolluted rain 40 years ago. Assume that carbonic acid (H2 CO3) formed by the reaction of CO2 and water is the only factor influencing pH. CO2(9) + H2O(1) H2CO3 (aq) Express your answer using one decimal place. hp ang Private Lodustrial Arca, e city,Chins 12. insert 81 64 4
8 leonline.hccs.edu/courses/188795/assignments/3601808 Homework Ch16 - Attempt 1 roblem 16.119 5 of 15 > Constants | Periodic Table Atmospheric CO2 levels have risen by nearly 20% over the past 40 years from 315 ppm to 380 ppm. What volume of CO2 at 25°C and 1.0 atm is dissolved in a 18.0-L bucket of today's rainwater? Express your answer using two significant figures. Vco, hp Leve Flectroaica Co, Lad ting Shicboog Privae lndustrial Acca, Tova Dongguaa ciy,Chins ADVA f12. fs insert 84 64 4 V. 5. 6. N Careers Mail - boss Texas Work HOc HomewcX W College Ph + eonline.hccs.edu/courses/188795/assignments/3601808 omework Ch16 - Attempt 1 oblem 16.119 <> 05 of 15 > Constants | Periodic Table Part A Atmospheric CO2 levels have risen by nearly 20% over the past 40 years from 315 ppm to 380 ppm. Given that the average pH of clean, unpolluted rain today is 5.4, determine the pH of unpolluted rain 40 years ago. Assume that carbonic acid (H2 CO3) formed by the reaction of CO2 and water is the only factor influencing pH. CO2(9) + H2O(1) H2CO3 (aq) Express your answer using one decimal place. hp ang Private Lodustrial Arca, e city,Chins 12. insert 81 64 4
Chapter27: Molecular Fluorescence Spectroscopy
Section: Chapter Questions
Problem 27.11QAP
Related questions
Question
Transcribed Image Text:8
leonline.hccs.edu/courses/188795/assignments/3601808
Homework Ch16 - Attempt 1
roblem 16.119
5 of 15 >
Constants | Periodic Table
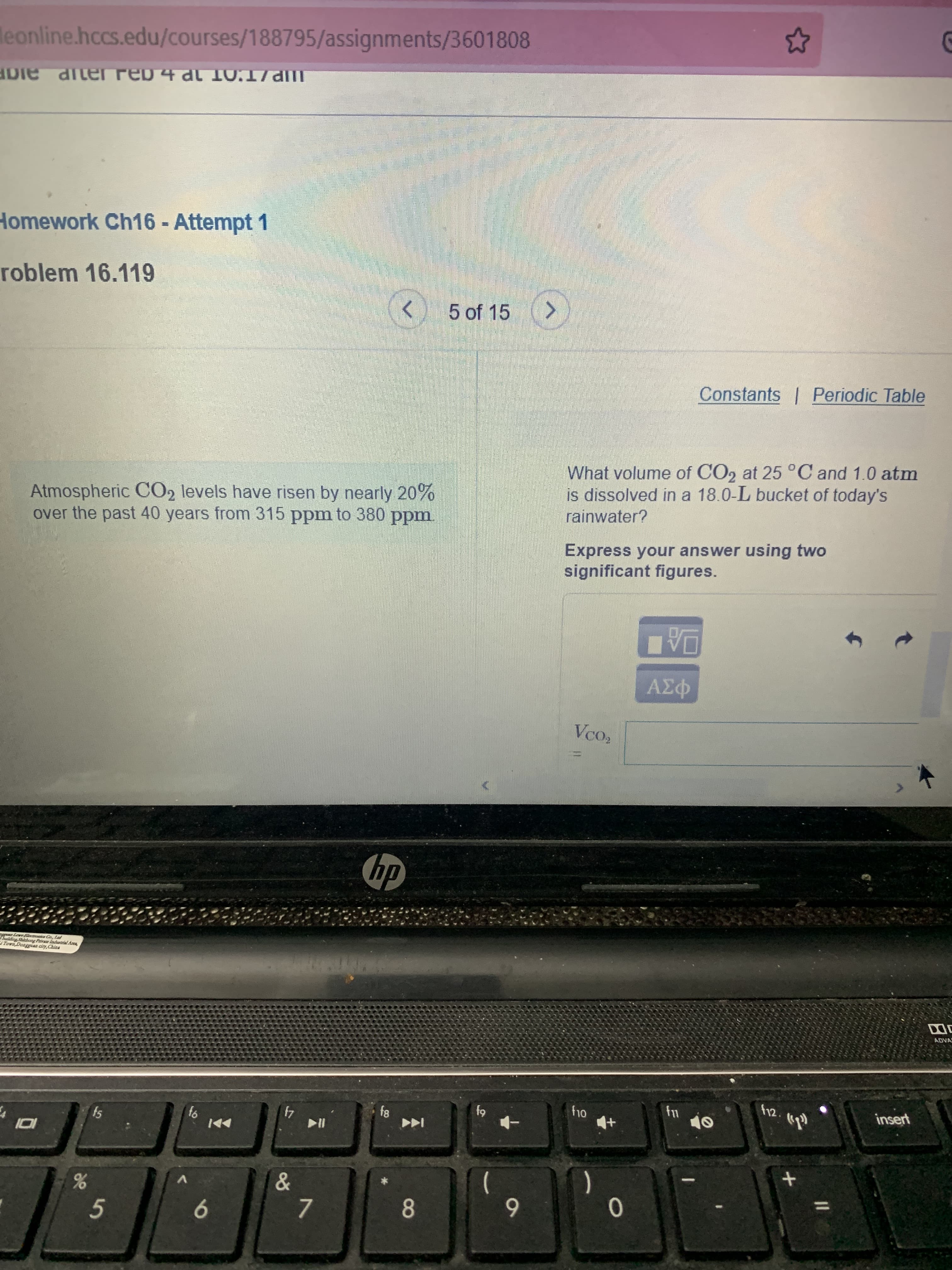
Atmospheric CO2 levels have risen by nearly 20%
over the past 40 years from 315 ppm to 380 ppm.
What volume of CO2 at 25°C and 1.0 atm
is dissolved in a 18.0-L bucket of today's
rainwater?
Express your answer using two
significant figures.
Vco,
hp
Leve Flectroaica Co, Lad
ting Shicboog Privae lndustrial Acca,
Tova Dongguaa ciy,Chins
ADVA
f12.
fs
insert
84
64
4
V.
5.
6.
Transcribed Image Text:N Careers
Mail - boss
Texas Work
HOc HomewcX
W College Ph +
eonline.hccs.edu/courses/188795/assignments/3601808
omework Ch16 - Attempt 1
oblem 16.119
<>
05 of 15 >
Constants | Periodic Table
Part A
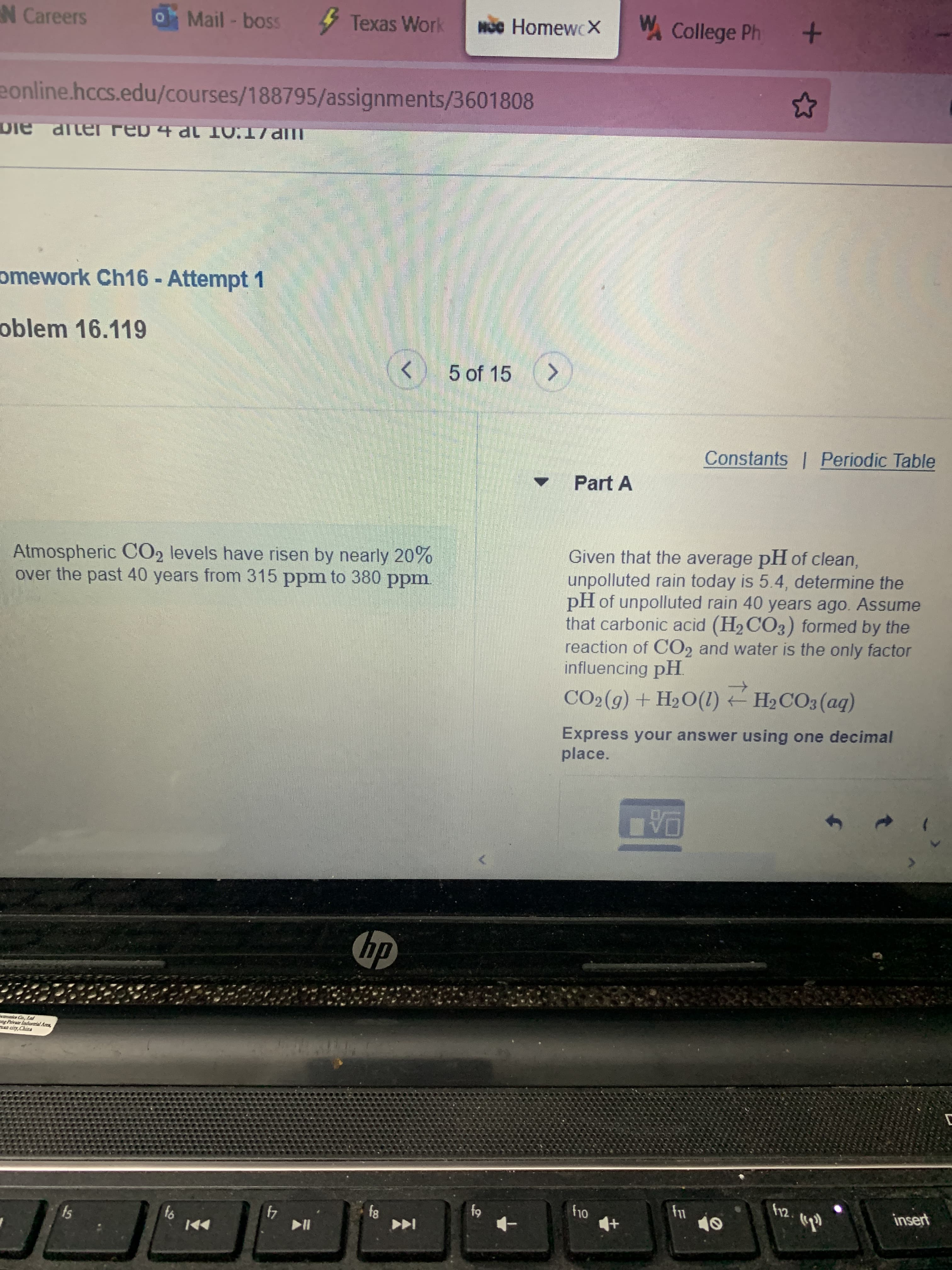
Atmospheric CO2 levels have risen by nearly 20%
over the past 40 years from 315 ppm to 380 ppm.
Given that the average pH of clean,
unpolluted rain today is 5.4, determine the
pH of unpolluted rain 40 years ago. Assume
that carbonic acid (H2 CO3) formed by the
reaction of CO2 and water is the only factor
influencing pH.
CO2(9) + H2O(1) H2CO3 (aq)
Express your answer using one decimal
place.
hp
ang Private Lodustrial Arca,
e city,Chins
12.
insert
81
64
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

