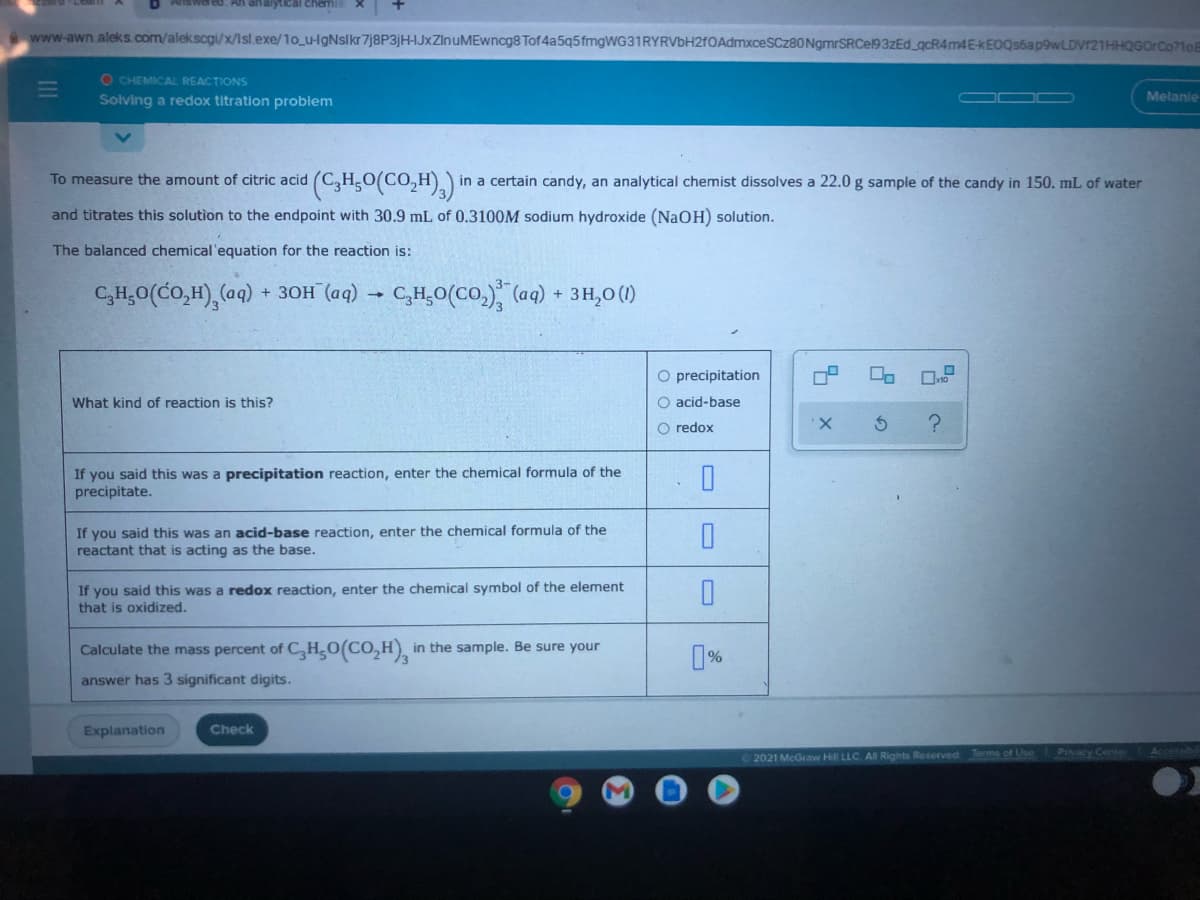
(CH,O(CO,H),)' To measure the amount of citric acid in a certain candy, an analytical chemist dissolves a 22.0 g sample of the candy in 150. mL of water and titrates this solution to the endpoint with 30.9 mL of 0.3100M sodium hydroxide (NaOH) solution. The balanced chemical'equation for the reaction is: C,H,0(CO,H), (aq) + ЗОН (ад) CH,0(CO.), (aq) + 3H,0 () O precipitation What kind of reaction is this? O acid-base O redox X. If you said this was a precipitation reaction, enter the chemical formula of the precipitate. If you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base. If you said this was a redox reaction, enter the chemical symbol of the element that is oxidized. Calculate the mass percent of C,H,O(CO,H) in the sample. Be sure your answer has 3 significant digits.
(CH,O(CO,H),)' To measure the amount of citric acid in a certain candy, an analytical chemist dissolves a 22.0 g sample of the candy in 150. mL of water and titrates this solution to the endpoint with 30.9 mL of 0.3100M sodium hydroxide (NaOH) solution. The balanced chemical'equation for the reaction is: C,H,0(CO,H), (aq) + ЗОН (ад) CH,0(CO.), (aq) + 3H,0 () O precipitation What kind of reaction is this? O acid-base O redox X. If you said this was a precipitation reaction, enter the chemical formula of the precipitate. If you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base. If you said this was a redox reaction, enter the chemical symbol of the element that is oxidized. Calculate the mass percent of C,H,O(CO,H) in the sample. Be sure your answer has 3 significant digits.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.6QAP
Related questions
Question
Hey if you could answer all the parts of this question it would be greatly appreciated!
Transcribed Image Text:Anawel ed. Ai an alytical chemis
www-awn aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr 7j8P3jH-IJxZInuMEwncg8 Tof 4a5q5fmgWG31RYRVbH2fOAdmxceSCz80NgmrSRCel93zEd_gcR4m4E-KEOQs6ap9wLDVr21HHQGOrCo710E
O CHEMICAL REACTIONS
Melanie
Solving a redox titration problem
To measure the amount of citric acid (C,H,0(CO,H).) in a certain candy, an analytical chemist dissolves a 22.0 g sample of the candy in 150. mL of water
and titrates this solution to the endpoint with 30.9 mL of 0.3100M sodium hydroxide (NaOH) solution.
The balanced chemical'equation for the reaction is:
C,H,0(CO,H),(aq)
+ ЗОН (аq)
C,H,0(CO.) (aq) + 3H,0 (1)
O precipitation
What kind of reaction is this?
O acid-base
O redox
If you said this was a precipitation reaction, enter the chemical formula of the
precipitate.
If you said this was an acid-base reaction, enter the chemical formula of the
reactant that is acting as the base.
If you said this was a redox reaction, enter the chemical symbol of the element
that is oxidized.
Calculate the mass percent of C,H,O(CO,H),
in the sample. Be sure your
answer has 3 significant digits.
Explanation
Check
Pavacy Cente
Access
2021 McGraw Hill LLC All Rights Reserved Terms of Use
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning