cl4 undergoes first order radioactive decay. For simplicity let its half-life be 6,000 years. The disintegration rate, measured as counts/sec by a radiation detector, is proportional to the amount of C1ª remaining. Suppose a sample which originally showed 8,000 counts/sec now shows 1000 counts/sec. What is the age of the sample? Note that you can't put a comma in your answer. years
cl4 undergoes first order radioactive decay. For simplicity let its half-life be 6,000 years. The disintegration rate, measured as counts/sec by a radiation detector, is proportional to the amount of C1ª remaining. Suppose a sample which originally showed 8,000 counts/sec now shows 1000 counts/sec. What is the age of the sample? Note that you can't put a comma in your answer. years
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter21: Nuclear Chemistry
Section: Chapter Questions
Problem 21.40QE
Related questions
Question
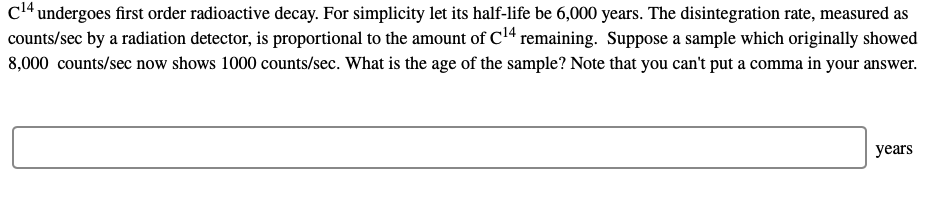
Transcribed Image Text:cl4 undergoes first order radioactive decay. For simplicity let its half-life be 6,000 years. The disintegration rate, measured as
counts/sec by a radiation detector, is proportional to the amount of C1ª remaining. Suppose a sample which originally showed
8,000 counts/sec now shows 1000 counts/sec. What is the age of the sample? Note that you can't put a comma in your answer.
years
Expert Solution
Step 1

Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning