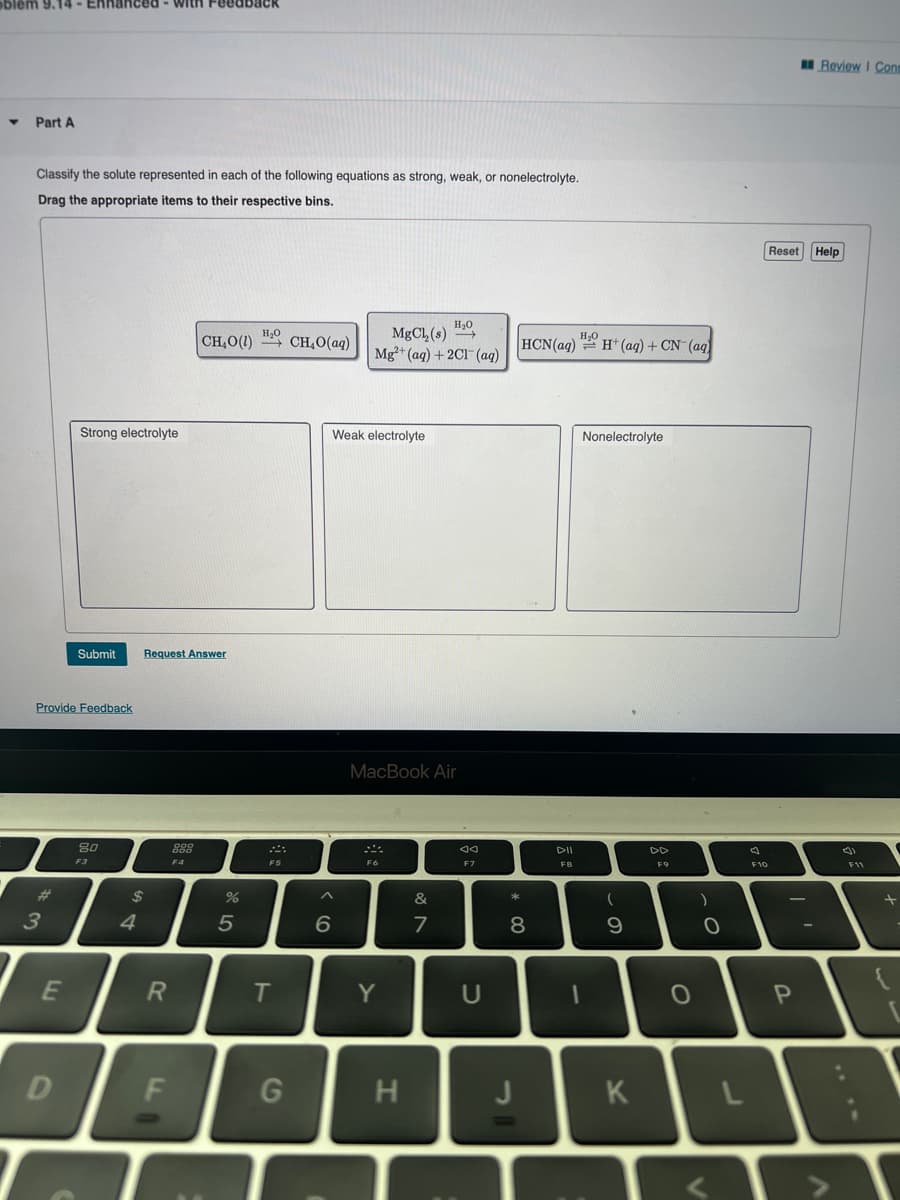
Classify the solute represented in each of the following equations as strong, weak, or nonelectrolyte. Drag the appropriate items to their respective bins. Strong electrolyte | CH,O(1) H₂O CH₂O(aq) H₂O MgCl,(s) ""} Mg²+ (aq) + 2Cl(aq) Weak electrolyte H₂O HCN(aq) H+ (aq) + CN- (aq) Nonelectrolyte Reset Help
Classify the solute represented in each of the following equations as strong, weak, or nonelectrolyte. Drag the appropriate items to their respective bins. Strong electrolyte | CH,O(1) H₂O CH₂O(aq) H₂O MgCl,(s) ""} Mg²+ (aq) + 2Cl(aq) Weak electrolyte H₂O HCN(aq) H+ (aq) + CN- (aq) Nonelectrolyte Reset Help
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.26QAP
Related questions
Question
Transcribed Image Text:blem 9.14- Enhanced with Feedback
▼
Part A
Classify the solute represented in each of the following equations as strong, weak, or nonelectrolyte.
Drag the appropriate items to their respective bins.
3
Provide Feedback
E
Strong electrolyte
D
Submit
80
F3
$
4
Request Answer
R
F
F4
CH,O() “}CH,O(aq)
%
H₂O
5
F5
T
G
Weak electrolyte
^
H₂O
MgCl₂ (s) →→→
Mg2+ (aq) + 2Cl(aq)
6
MacBook Air
F6
Y
H
&
7
Aa
U
*
HCN(aq)
8
į
F8
H₂H+ (aq) + CN- (aq)
Nonelectrolyte
1
(
9
K
F9
O
)
O
A
F10
Reset Help
P
-
Review I Con
니
V
(4)
F11
+
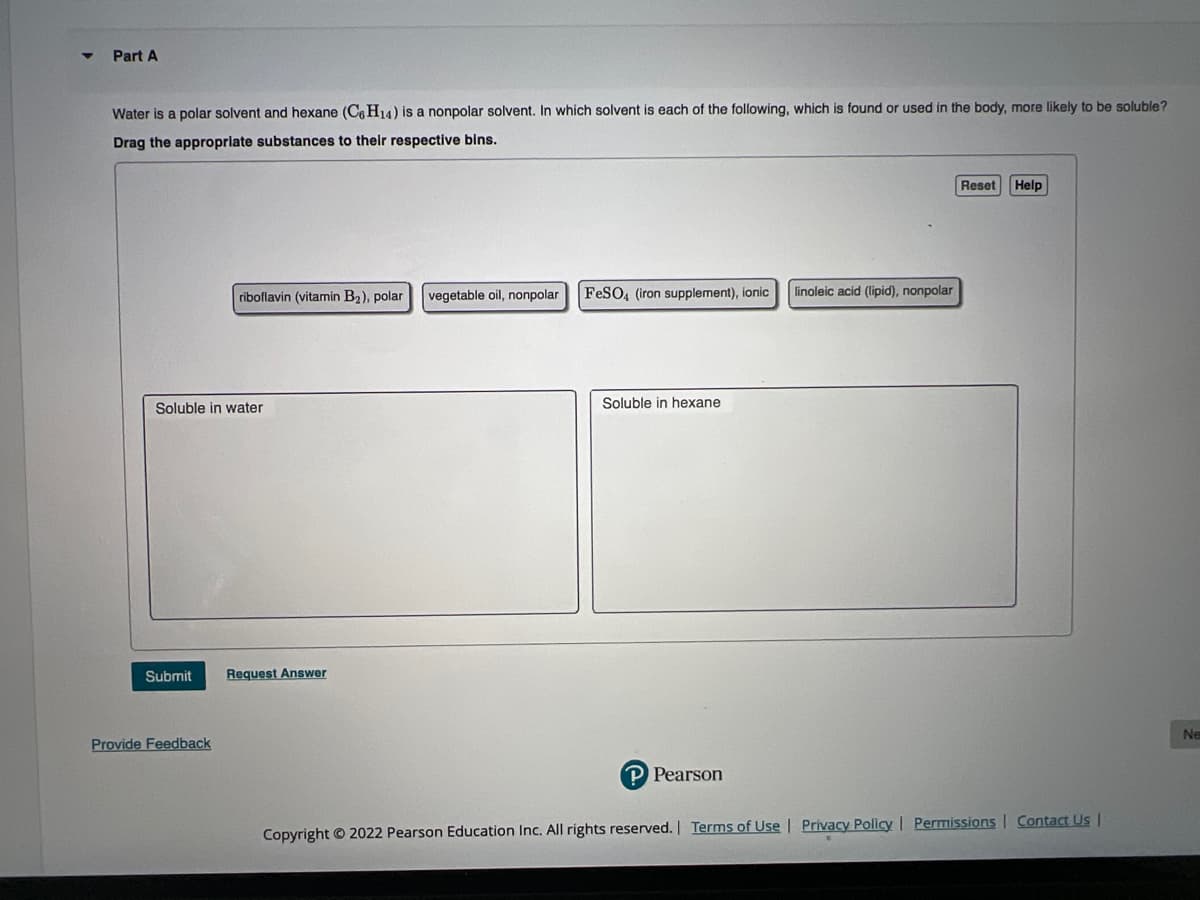
Transcribed Image Text:Part A
Water is a polar solvent and hexane (C6H14) is a nonpolar solvent. In which solvent is each of the following, which is found or used in the body, more likely to be soluble?
Drag the appropriate substances to their respective bins.
riboflavin (vitamin B₂), polar
Soluble in water
Submit Request Answer
Provide Feedback
vegetable oil, nonpolar
FeSO4 (iron supplement), ionic
Soluble in hexane
P Pearson
linoleic acid (lipid), nonpolar
Reset Help
Copyright © 2022 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions | Contact Us |
Ne
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning