clear liquid CuSO4 Copper(I) B2 NH4OH Aqueous light blue liq became a da yes Ammonia Sulfate B3 Pb(NO32 Lead clear liquid CaCl2 Calcium clear liquid turned to a w yes Nitrate Chloride B4 NaHSO4 in the beginni yes clear liquid clear liquid CaCl2 Calcium Sodium Bisulfate Chloride В5 NAHSO4 Na cO3 Sodium bubbled whe clear liquid clear liquid yes Sodium Bisulfate Carbonate CuSO4 В6 light blue liq NAHCO3 clear liquid light blue liqu no Copper(II) Sulfate Sodium Bicarbonate Blue Dye #1 C1 НC dark blue liq clear liquid turned into al yes Hydrochloric Acid C2 Na2CO3 Sodium C20H1404 Phenolphthalein clear liquid clear liquid looks the san yes Carbonate Data Table 2: Reaction Observations Observations Chemical Well Chemical #1 (4 Chemical Chemical #2 (4 drops) Chemical drops) #1 #2 Change (Yes/No) Appearance Appearance A1 NaHCOз HC chemical is chemical is bubbled and yes Sodium Hydrochloric Acid Bicarbonate A2 IKI indicator it is a red/bn Starch cloudy immediate ch yes Pb(NO3}2 Lead() Nitrate АЗ KI Potassium clear liquid immediately t yes clear liquid lodide A4 NaOH Sodium C20H1404 Phenolphthalein clear liquid clear liquid immediately t yes Hydroxide А5 HC C20H1404 Phenolphthalein clear liquid clear liquid no change-cl no Hydrochloric Acid A6 NAOH Sodium AgNO3 Silver(I) clear liquid brown grainy yes clear liquid Hydroxide Nitrate B1 AgNO3 Silver NH2OH Aqueous clear liquid clear liquid Observation no Nitrate Ammonia 1:
clear liquid CuSO4 Copper(I) B2 NH4OH Aqueous light blue liq became a da yes Ammonia Sulfate B3 Pb(NO32 Lead clear liquid CaCl2 Calcium clear liquid turned to a w yes Nitrate Chloride B4 NaHSO4 in the beginni yes clear liquid clear liquid CaCl2 Calcium Sodium Bisulfate Chloride В5 NAHSO4 Na cO3 Sodium bubbled whe clear liquid clear liquid yes Sodium Bisulfate Carbonate CuSO4 В6 light blue liq NAHCO3 clear liquid light blue liqu no Copper(II) Sulfate Sodium Bicarbonate Blue Dye #1 C1 НC dark blue liq clear liquid turned into al yes Hydrochloric Acid C2 Na2CO3 Sodium C20H1404 Phenolphthalein clear liquid clear liquid looks the san yes Carbonate Data Table 2: Reaction Observations Observations Chemical Well Chemical #1 (4 Chemical Chemical #2 (4 drops) Chemical drops) #1 #2 Change (Yes/No) Appearance Appearance A1 NaHCOз HC chemical is chemical is bubbled and yes Sodium Hydrochloric Acid Bicarbonate A2 IKI indicator it is a red/bn Starch cloudy immediate ch yes Pb(NO3}2 Lead() Nitrate АЗ KI Potassium clear liquid immediately t yes clear liquid lodide A4 NaOH Sodium C20H1404 Phenolphthalein clear liquid clear liquid immediately t yes Hydroxide А5 HC C20H1404 Phenolphthalein clear liquid clear liquid no change-cl no Hydrochloric Acid A6 NAOH Sodium AgNO3 Silver(I) clear liquid brown grainy yes clear liquid Hydroxide Nitrate B1 AgNO3 Silver NH2OH Aqueous clear liquid clear liquid Observation no Nitrate Ammonia 1:
Chapter13: Isolation Of Eugenol From Clov
Section: Chapter Questions
Problem 9Q
Related questions
Question
100%
Data Table 2 contains nine double displacement reactions. Write a balanced chemical equation for five of those reactions.
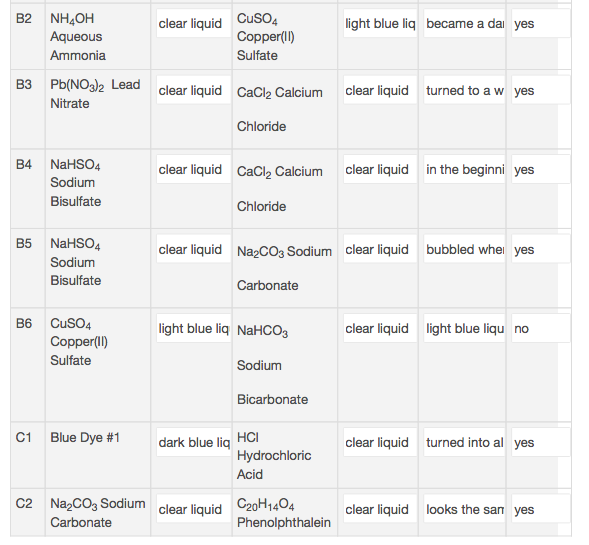
Transcribed Image Text:clear liquid CuSO4
Copper(I)
B2 NH4OH
Aqueous
light blue liq
became a da yes
Ammonia
Sulfate
B3 Pb(NO32 Lead clear liquid CaCl2 Calcium
clear liquid
turned to a w yes
Nitrate
Chloride
B4 NaHSO4
in the beginni yes
clear liquid
clear liquid
CaCl2 Calcium
Sodium
Bisulfate
Chloride
В5
NAHSO4
Na cO3 Sodium
bubbled whe
clear liquid
clear liquid
yes
Sodium
Bisulfate
Carbonate
CuSO4
В6
light blue liq NAHCO3
clear liquid
light blue liqu no
Copper(II)
Sulfate
Sodium
Bicarbonate
Blue Dye #1
C1
НC
dark blue liq
clear liquid
turned into al yes
Hydrochloric
Acid
C2
Na2CO3 Sodium
C20H1404
Phenolphthalein
clear liquid
clear liquid
looks the san yes
Carbonate
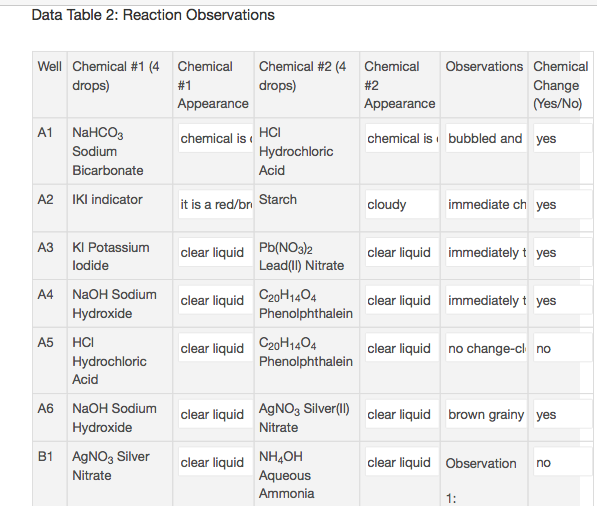
Transcribed Image Text:Data Table 2: Reaction Observations
Observations Chemical
Well Chemical #1 (4 Chemical
Chemical #2 (4
drops)
Chemical
drops)
#1
#2
Change
(Yes/No)
Appearance
Appearance
A1 NaHCOз
HC
chemical is
chemical is bubbled and yes
Sodium
Hydrochloric
Acid
Bicarbonate
A2
IKI indicator
it is a red/bn Starch
cloudy
immediate ch yes
Pb(NO3}2
Lead() Nitrate
АЗ
KI Potassium
clear liquid immediately t yes
clear liquid
lodide
A4
NaOH Sodium
C20H1404
Phenolphthalein
clear liquid
clear liquid immediately t yes
Hydroxide
А5
HC
C20H1404
Phenolphthalein
clear liquid
clear liquid no change-cl no
Hydrochloric
Acid
A6
NAOH Sodium
AgNO3 Silver(I)
clear liquid brown grainy yes
clear liquid
Hydroxide
Nitrate
B1 AgNO3 Silver
NH2OH
Aqueous
clear liquid
clear liquid Observation
no
Nitrate
Ammonia
1:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT