Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 13CQ: Most materials expand when heated. One notable exception is water between 0 and 4 , which actually...
Related questions
Question
100%
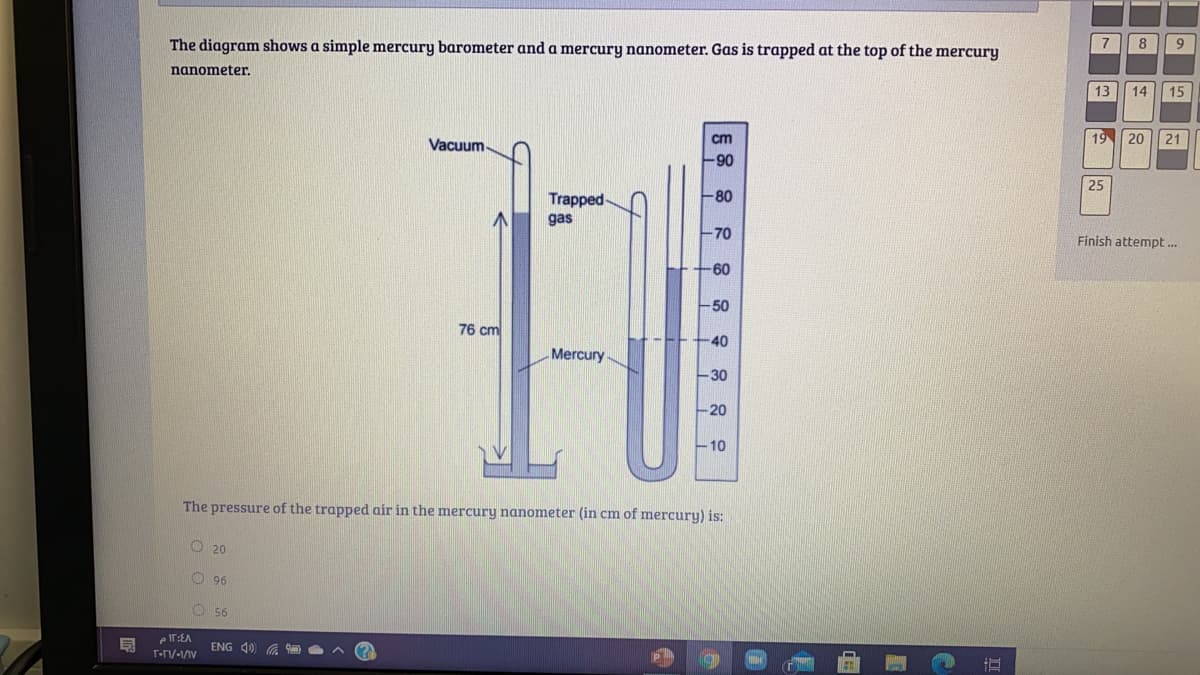
Transcribed Image Text:The diagram shows a simple mercury barometer and a mercury nanometer. Gas is trapped at the top of the mercury
7
8
nanometer.
13
14
15
cm
19
20
21
Vacuum-
90
25
Trapped-
-80
gas
-70
Finish attempt..
-60
-50
76 cm
40
Mercury
-30
-20
10
The pressure of the trapped air in the mercury nanometer (in cm of mercury) is:
O 20
O 96
O 56
e IT:EA
ENG 40 G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Stars and Galaxies
Physics
ISBN:
9781305120785
Author:
Michael A. Seeds, Dana Backman
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning


Stars and Galaxies
Physics
ISBN:
9781305120785
Author:
Michael A. Seeds, Dana Backman
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College