Coenzyme A, NAD*, and FAD are coenzymes that are necessary for energy production. Classify each statement as describing coenzyme A, NAD+, or FAD. Coenzyme A NAD+ FAD Answer Bank accepts two hydrogen atoms when it is reduced derived from the vitamin pantothenic acid (B₂) accepts two electrons and one proton when it is reduced derived from the vitamin nicotinamide derived from the vitamin riboflavin (B₂) forms part of acetyl-SCOA, which is part of the citric acid cycle
Coenzyme A, NAD*, and FAD are coenzymes that are necessary for energy production. Classify each statement as describing coenzyme A, NAD+, or FAD. Coenzyme A NAD+ FAD Answer Bank accepts two hydrogen atoms when it is reduced derived from the vitamin pantothenic acid (B₂) accepts two electrons and one proton when it is reduced derived from the vitamin nicotinamide derived from the vitamin riboflavin (B₂) forms part of acetyl-SCOA, which is part of the citric acid cycle
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter24: Carbohydrate Metabolism
Section: Chapter Questions
Problem 24.117EP
Related questions
Concept explainers
Organic Chemistry of Metabolic Pathways
Metabolic pathways allude to the arrangement of chemical catalyzed reactions that lead to the transformation of a substance into the final product. Metabolic pathways incorporate a progression of reaction where the substrate is changed continuously and the transitional metabolites are persistently recovered.
Glucogenesis
Glucogenesis is a metabolic pathway in which glucose is produced from carbon substrates that are not carbohydrates. This process is observed in plants, animals, fungi, bacteria and other micro organisms. The general definition for glucogenesis or gluconeogenesis is as follows,
Question
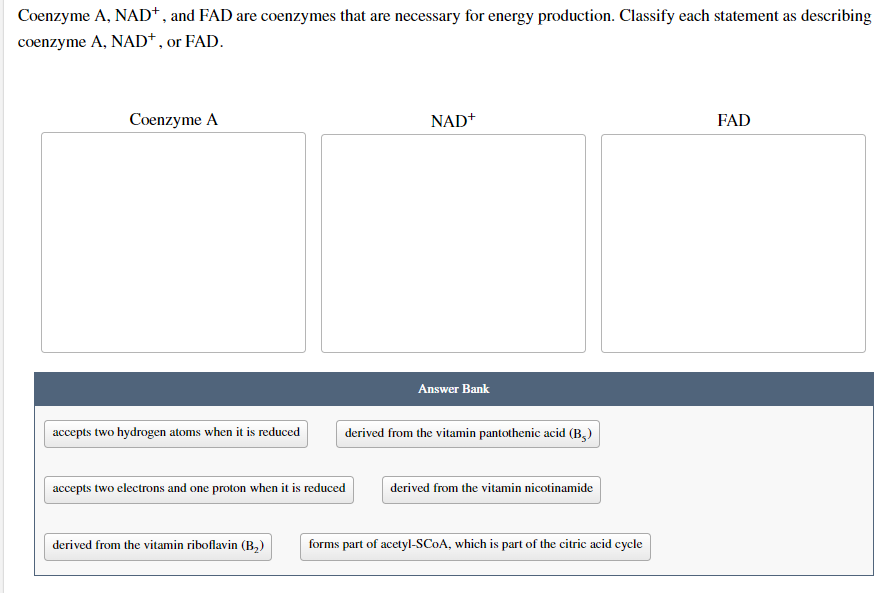
Transcribed Image Text:Coenzyme A, NAD*, and FAD are coenzymes that are necessary for energy production. Classify each statement as describing
coenzyme A, NAD+, or FAD.
Coenzyme A
NAD+
FAD
Answer Bank
accepts two hydrogen atoms when it is reduced
derived from the vitamin pantothenic acid (B₂)
accepts two electrons and one proton when it is reduced
derived from the vitamin nicotinamide
derived from the vitamin riboflavin (B₂)
forms part of acetyl-SCOA, which is part of the citric acid cycle
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
