Complete a net ionic equation for each proton-transfer reaction. NH4* + OH - Draw the formulas of the two products. • Draw cations and anions in separate sketchers. Separate structures with + signs from the drop-down menu. ● 0- 90-85 soll H-N H CH3 ChemDoodle Sn [F +v D 0- ▾ **** E H-O-H
Complete a net ionic equation for each proton-transfer reaction. NH4* + OH - Draw the formulas of the two products. • Draw cations and anions in separate sketchers. Separate structures with + signs from the drop-down menu. ● 0- 90-85 soll H-N H CH3 ChemDoodle Sn [F +v D 0- ▾ **** E H-O-H
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section14.9: Lewis Acids And Bases
Problem 14.26E
Related questions
Question
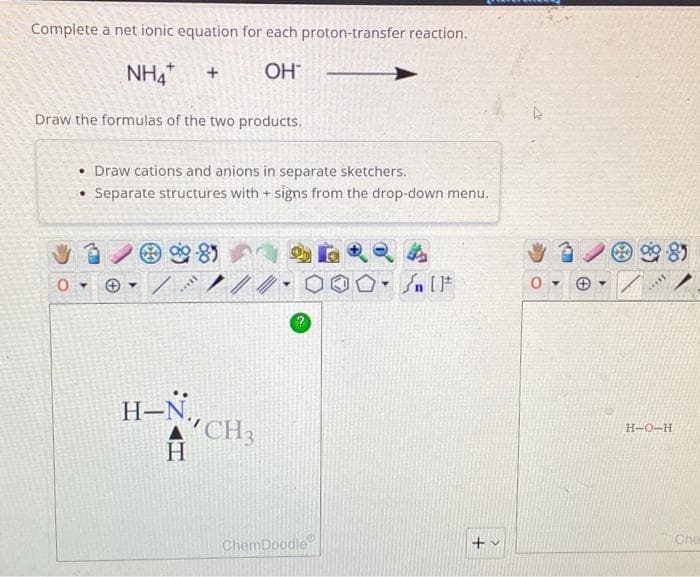
Transcribed Image Text:Complete a net ionic equation for each proton-transfer reaction.
NHA +
OH™
Draw the formulas of the two products.
0
• Draw cations and anions in separate sketchers.
• Separate structures with + signs from the drop-down menu.
▾
soll
H-N,
H
CH3
Y
ChemDoodle
DRQA
Sn [F
0-
▾
98)
***
H-O-H
Che
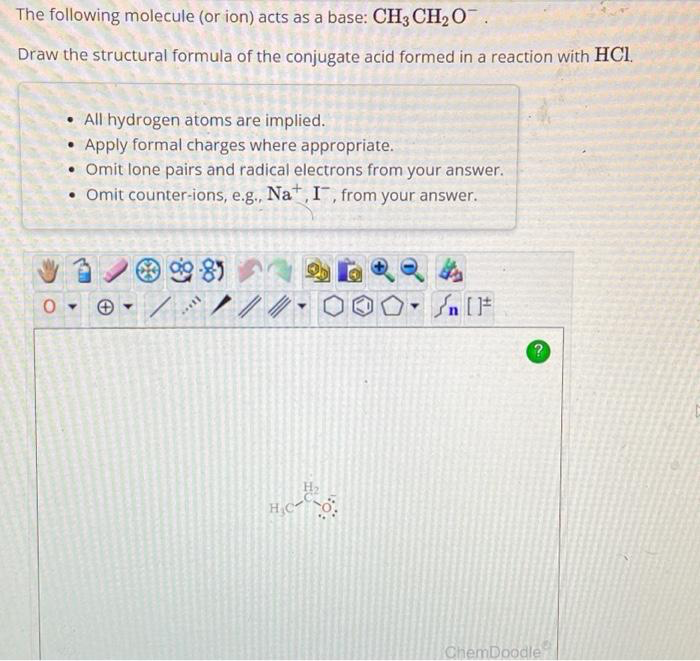
Transcribed Image Text:The following molecule (or ion) acts as a base: CH3 CH₂O.
Draw the structural formula of the conjugate acid formed in a reaction with HCl.
O
All hydrogen atoms are implied.
Apply formal charges where appropriate.
. Omit lone pairs and radical electrons from your answer.
• Omit counter-ions, e.g., Na+, I, from your answer.
**
//,
H₂CO
Sn [F
?
ChemDoodle
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning