Complete combustion of 8.40 g of a hydrocarbon produced 25.9 g of CO, and 11.9 g of H,O. What is the empirical formula for the hydrocarbon? Insert subscripts as necessary. empirical formula: CH
Complete combustion of 8.40 g of a hydrocarbon produced 25.9 g of CO, and 11.9 g of H,O. What is the empirical formula for the hydrocarbon? Insert subscripts as necessary. empirical formula: CH
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter10: The Mole
Section: Chapter Questions
Problem 1STP
Related questions
Question
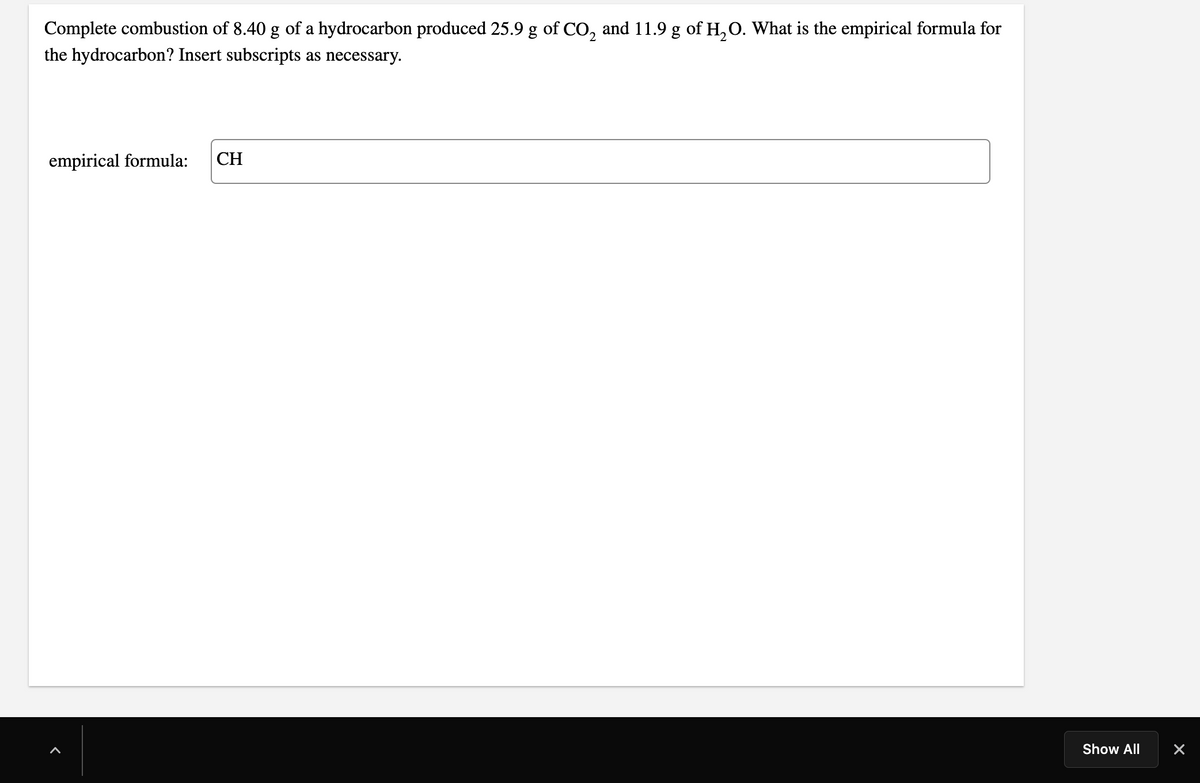
Transcribed Image Text:Complete combustion of 8.40 g of a hydrocarbon produced 25.9 g of CO, and 11.9 g of H,O. What is the empirical formula for
the hydrocarbon? Insert subscripts as necessary.
empirical formula:
CH
Show All
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning