Comprehension Check #5: a) If a rock has 25% Potassium-40, what is the proportion of Argon-40? b) How many half-lives have elapsed? What is the age of the rock?
Comprehension Check #5: a) If a rock has 25% Potassium-40, what is the proportion of Argon-40? b) How many half-lives have elapsed? What is the age of the rock?
Applications and Investigations in Earth Science (9th Edition)
9th Edition
ISBN:9780134746241
Author:Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Chapter1: The Study Of Minerals
Section: Chapter Questions
Problem 1LR
Related questions
Question
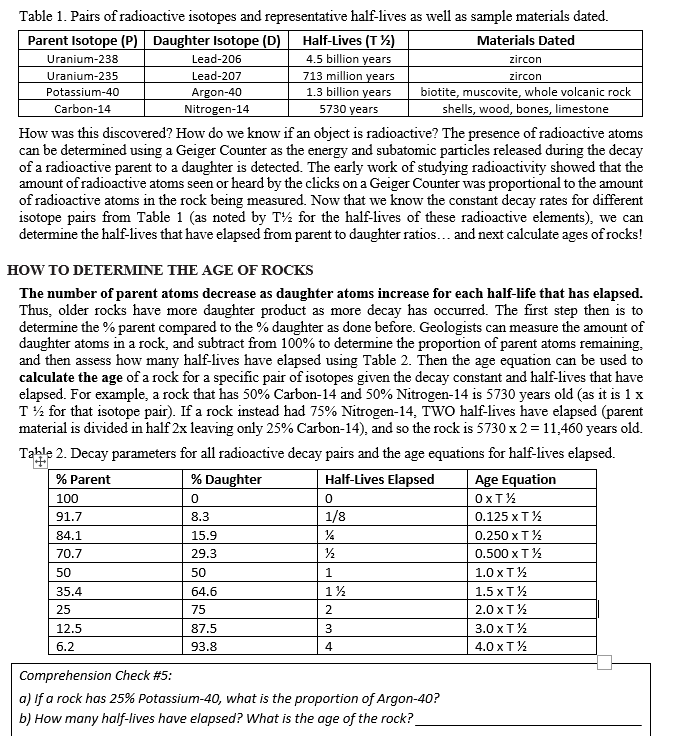
Transcribed Image Text:Table 1. Pairs of radioactive isotopes and representative half-lives as well as sample materials dated.
Parent Isotope (P) Daughter Isotope (D)
Half-Lives (T ½)
Materials Dated
Uranium-238
Uranium-235
4.5 billion years
713 million years
1.3 billion years
5730 years
Potassium-40
Carbon-14
Lead-206
Lead-207
Argon-40
Nitrogen-14
How was this discovered? How do we know if an object is radioactive? The presence of radioactive atoms
can be determined using a Geiger Counter as the energy and subatomic particles released during the decay
of a radioactive parent to a daughter is detected. The early work of studying radioactivity showed that the
amount of radioactive atoms seen or heard by the clicks on a Geiger Counter was proportional to the amount
of radioactive atoms in the rock being measured. Now that we know the constant decay rates for different
isotope pairs from Table 1 (as noted by T for the half-lives of these radioactive elements), we can
determine the half-lives that have elapsed from parent to daughter ratios... and next calculate ages of rocks!
% Daughter
0
8.3
HOW TO DETERMINE THE AGE OF ROCKS
The number of parent atoms decrease as daughter atoms increase for each half-life that has elapsed.
Thus, older rocks have more daughter product as more decay has occurred. The first step then is to
determine the % parent compared to the % daughter as done before. Geologists can measure the amount of
daughter atoms in a rock, and subtract from 100% to determine the proportion of parent atoms remaining.
and then assess how many half-lives have elapsed using Table 2. Then the age equation can be used to
calculate the age of a rock for a specific pair of isotopes given the decay constant and half-lives that have
elapsed. For example, a rock that has 50% Carbon-14 and 50% Nitrogen-14 is 5730 years old (as it is 1 x
T ½ for that isotope pair). If a rock instead had 75% Nitrogen-14, TWO half-lives have elapsed (parent
material is divided in half 2x leaving only 25% Carbon-14), and so the rock is 5730 x 2 = 11,460 years old.
Table 2. Decay parameters for all radioactive decay pairs and the age equations for half-lives elapsed.
% Parent
Half-Lives Elapsed
100
91.7
84.1
70.7
50
35.4
25
12.5
6.2
15.9
29.3
50
64.6
75
87.5
93.8
zircon
zircon
biotite, muscovite, whole volcanic rock
shells, wood, bones, limestone
0
1/8
½
½½
1
1½
2
3
4
Comprehension Check #5:
a) If a rock has 25% Potassium-40, what is the proportion of Argon-40?
b) How many half-lives have elapsed? What is the age of the rock?_
Age Equation
OXT½
0.125 x T ½
0.250 x T ½
0.500 x T ½
1.0 x T ½
1.5 x T ½
2.0 XT ¹2
3.0 x T ½
4.0 XT ½
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Applications and Investigations in Earth Science …
Earth Science
ISBN:
9780134746241
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Exercises for Weather & Climate (9th Edition)
Earth Science
ISBN:
9780134041360
Author:
Greg Carbone
Publisher:
PEARSON

Environmental Science
Earth Science
ISBN:
9781260153125
Author:
William P Cunningham Prof., Mary Ann Cunningham Professor
Publisher:
McGraw-Hill Education

Applications and Investigations in Earth Science …
Earth Science
ISBN:
9780134746241
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Exercises for Weather & Climate (9th Edition)
Earth Science
ISBN:
9780134041360
Author:
Greg Carbone
Publisher:
PEARSON

Environmental Science
Earth Science
ISBN:
9781260153125
Author:
William P Cunningham Prof., Mary Ann Cunningham Professor
Publisher:
McGraw-Hill Education

Earth Science (15th Edition)
Earth Science
ISBN:
9780134543536
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Environmental Science (MindTap Course List)
Earth Science
ISBN:
9781337569613
Author:
G. Tyler Miller, Scott Spoolman
Publisher:
Cengage Learning

Physical Geology
Earth Science
ISBN:
9781259916823
Author:
Plummer, Charles C., CARLSON, Diane H., Hammersley, Lisa
Publisher:
Mcgraw-hill Education,