Concerning the albedo principle, when it is ice melts, which means heat is reflected back into space. O warmer, more O colder, more colder, less warmer, less
Concerning the albedo principle, when it is ice melts, which means heat is reflected back into space. O warmer, more O colder, more colder, less warmer, less
Chapter4: Forces Between Particles
Section: Chapter Questions
Problem 4.78E
Related questions
Question
please answer both questions

Transcribed Image Text:Concerning the albedo principle, when it is
ice melts, which
means
heat is reflected back into space.
O warmer, more
O colder, more
colder, less
warmer, less
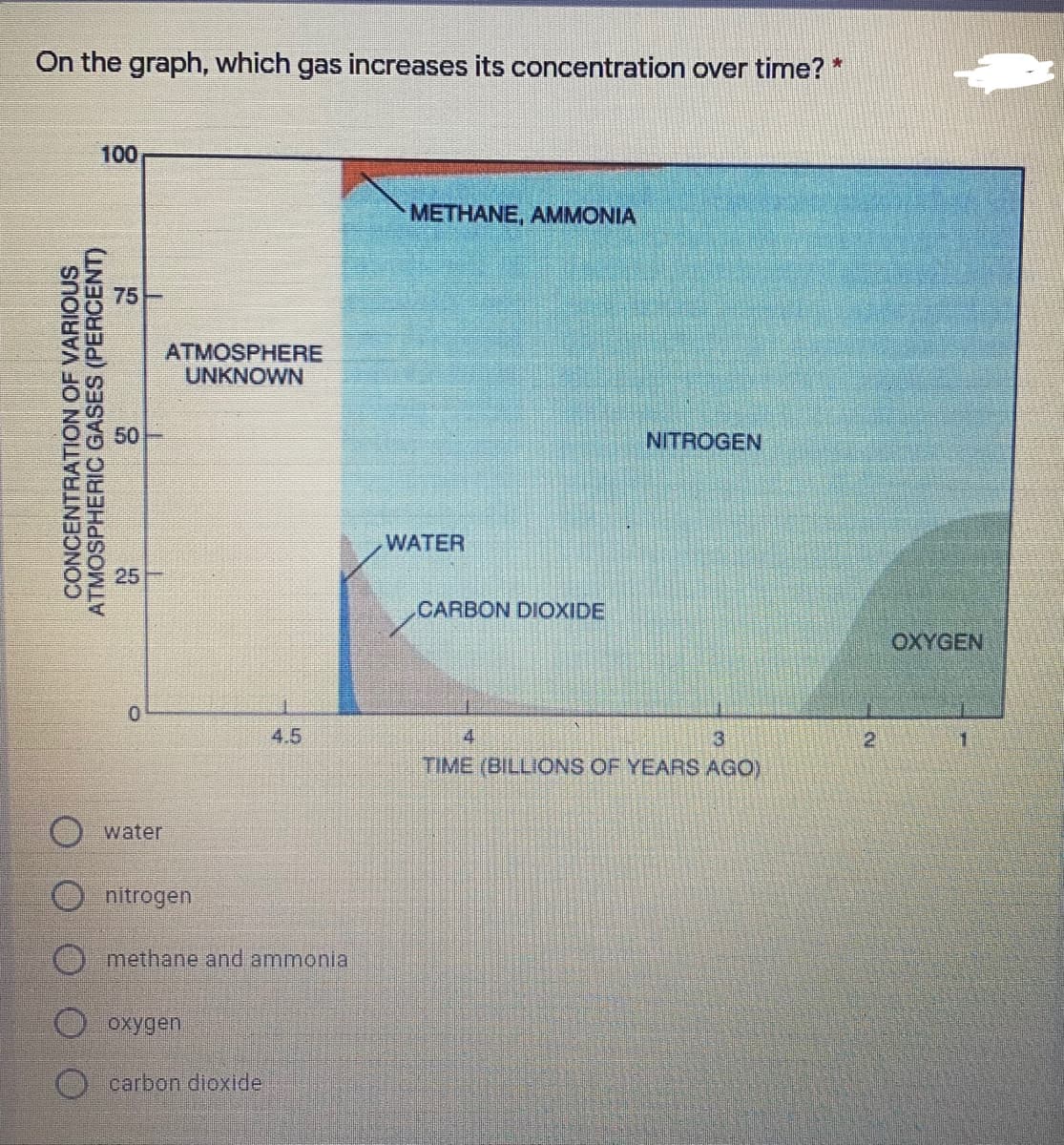
Transcribed Image Text:On the graph, which gas increases its concentration over time? *
100
METHANE, AMMONIA
75
ATMOSPHERE
UNKNOWN
NITROGEN
WATER
25
CARBON DIOXIDE
OXYGEN
4.5
4
3.
21
1.
TIME (BILLIONS OF YEARS AGO)
water
nitrogen
methane and ammonial
oxygen
carbon dioxide
CONCENTRATION OF VARIOUS
ATMOSPHERIC GASES (PERCENT)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning