Consider a heat engine consisting of two cylinders. Fuel is burned externally to warm one of the two cylinders, and n moles of an ideal monatomic gas (with molar heat capacity at constant volume Cy=3R/2) moves cyclically between the cylinders, expanding in the hot cylinder at temperature T2 and contracting in the cold cylinder at temperature T1. In a cycle of operation, the gas goes through the following sequence of steps: (i) Step ab: Isochoric heat transfer into the gas, making it change state from a at temperature T1 to b at temperature T2. (ii) Step bc: Isothermal expansion at temperature T, from state b with volume V to state c with volume rV, where r is a constant greater than unity. (iii) Step cd: Isochoric heat removal in going from state c at temperature T2 to state d at temperature T1. (iv) Step da: Isothermal compression at temperature T1 from state d with volume rV to state a with volume V. For this cycle, obtain the heat transfer to the engine Qh, the work done by the engine W, and the efficiency e.
Consider a heat engine consisting of two cylinders. Fuel is burned externally to warm one of the two cylinders, and n moles of an ideal monatomic gas (with molar heat capacity at constant volume Cy=3R/2) moves cyclically between the cylinders, expanding in the hot cylinder at temperature T2 and contracting in the cold cylinder at temperature T1. In a cycle of operation, the gas goes through the following sequence of steps: (i) Step ab: Isochoric heat transfer into the gas, making it change state from a at temperature T1 to b at temperature T2. (ii) Step bc: Isothermal expansion at temperature T, from state b with volume V to state c with volume rV, where r is a constant greater than unity. (iii) Step cd: Isochoric heat removal in going from state c at temperature T2 to state d at temperature T1. (iv) Step da: Isothermal compression at temperature T1 from state d with volume rV to state a with volume V. For this cycle, obtain the heat transfer to the engine Qh, the work done by the engine W, and the efficiency e.
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter1: Basic Modes Of Heat Transfer
Section: Chapter Questions
Problem 1.75P: Referring to Problem 1.74, how many kilograms of ice can a 3-ton refrigeration unit produce in a...
Related questions
Question
100%
Obtain the heat transfer to the engine Qh, the work done by the engine W, and the efficiency e.
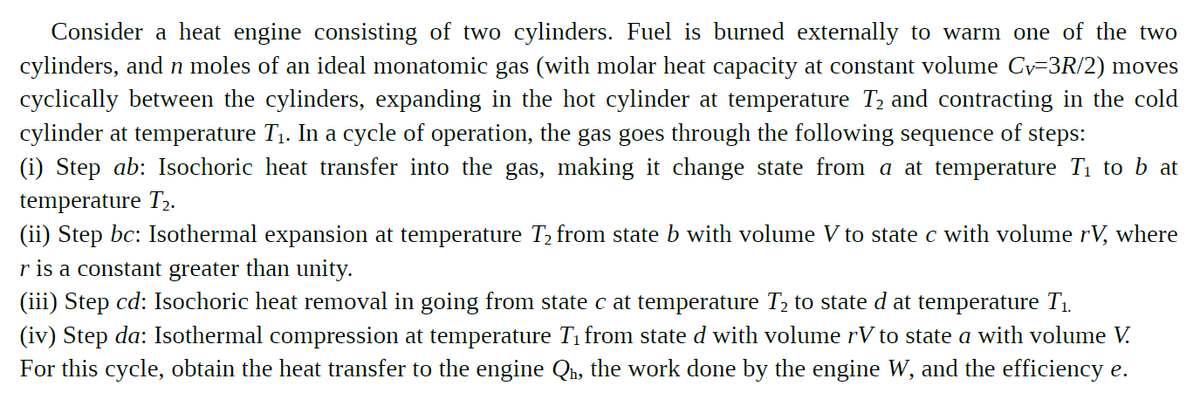
Transcribed Image Text:Consider a heat engine consisting of two cylinders. Fuel is burned externally to warm one of the two
cylinders, and n moles of an ideal monatomic gas (with molar heat capacity at constant volume Cy=3R/2) moves
cyclically between the cylinders, expanding in the hot cylinder at temperature T2 and contracting in the cold
cylinder at temperature T1. In a cycle of operation, the gas goes through the following sequence of steps:
(i) Step ab: Isochoric heat transfer into the gas, making it change state from a at temperature T1 to b at
temperature T,.
(ii) Step bc: Isothermal expansion at temperature T, from state b with volume V to state c with volume rV, where
r is a constant greater than unity.
(iii) Step cd: Isochoric heat removal in going from state c at temperature T2 to state d at temperature T1.
(iv) Step da: Isothermal compression at temperature Ti from state d with volume rV to state a with volume V.
For this cycle, obtain the heat transfer to the engine Qh, the work done by the engine W, and the efficiency e.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning