Consider a separation performed on a 40.0 mm long open tubular column with a 0.50 mm diameter and a 2,0 um thick stationary phase. Compound A eluted at 12.51 min, compound B eluted at 13.37 min, and the unretained solvent eluted at 1.145 min. What are the adjusted retention times, rh, and retention factors, k, for compounds A and B? min min
Consider a separation performed on a 40.0 mm long open tubular column with a 0.50 mm diameter and a 2,0 um thick stationary phase. Compound A eluted at 12.51 min, compound B eluted at 13.37 min, and the unretained solvent eluted at 1.145 min. What are the adjusted retention times, rh, and retention factors, k, for compounds A and B? min min
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter27: Gas Chromatography
Section: Chapter Questions
Problem 27.23QAP
Related questions
Question
1
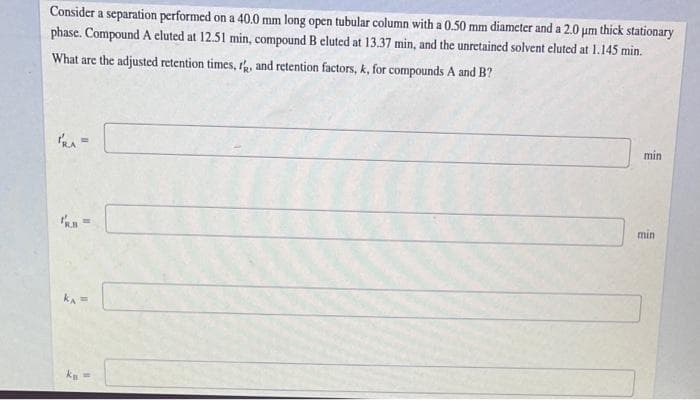
Transcribed Image Text:Consider a separation performed on a 40.0 mm long open tubular column with a 0.50 mm diameter and a 2,0 um thick stationary
phase. Compound A eluted at 12.51 min, compound B eluted at 13.37 min, and the unretained solvent eluted at 1.145 min.
What are the adjusted retention times, r, and retention factors, k, for compounds A and B?
min
min
k =
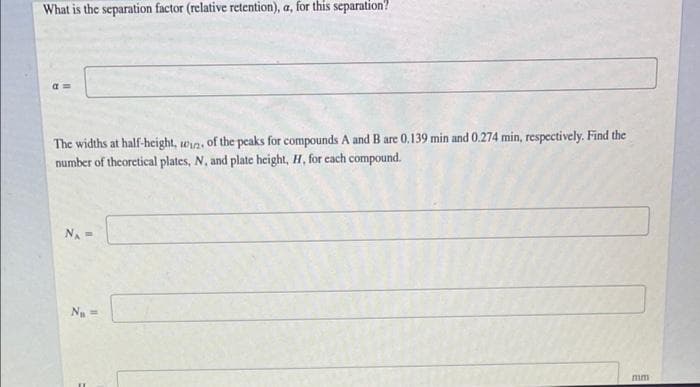
Transcribed Image Text:What is the separation factor (relative retention), a, for this separation?
The widths at half-height, win, of the peaks for compounds A and B are 0.139 min and 0.274 min, respectively. Find the
number of theoretical plates, N, and plate height, H, for cach compound.
NA
Na =
mm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

