Consider a U-shaped tube containing two aqueous solutions of ions, represented by orange spheres, separated by a semipermeable membrane at the center of the tube. Is the system shown in equilibrium? no ○ yes Which direction will the water flow? from right to left from left to right neither left nor right
Consider a U-shaped tube containing two aqueous solutions of ions, represented by orange spheres, separated by a semipermeable membrane at the center of the tube. Is the system shown in equilibrium? no ○ yes Which direction will the water flow? from right to left from left to right neither left nor right
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter18: Chemical Equilibrium
Section: Chapter Questions
Problem 3E
Related questions
Question
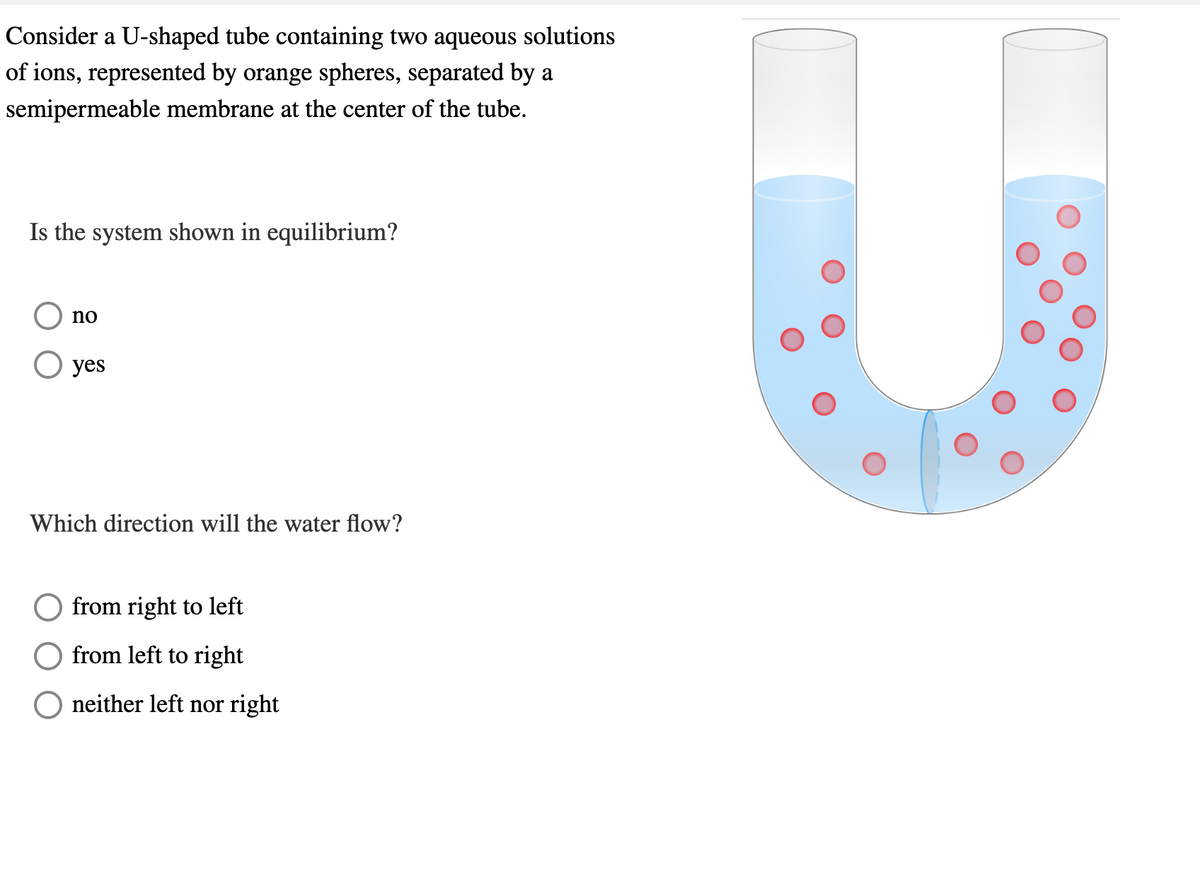
Transcribed Image Text:Consider a U-shaped tube containing two aqueous solutions
of ions, represented by orange spheres, separated by a
semipermeable membrane at the center of the tube.
Is the system shown in equilibrium?
no
○ yes
Which direction will the water flow?
from right to left
from left to right
neither left nor right
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning