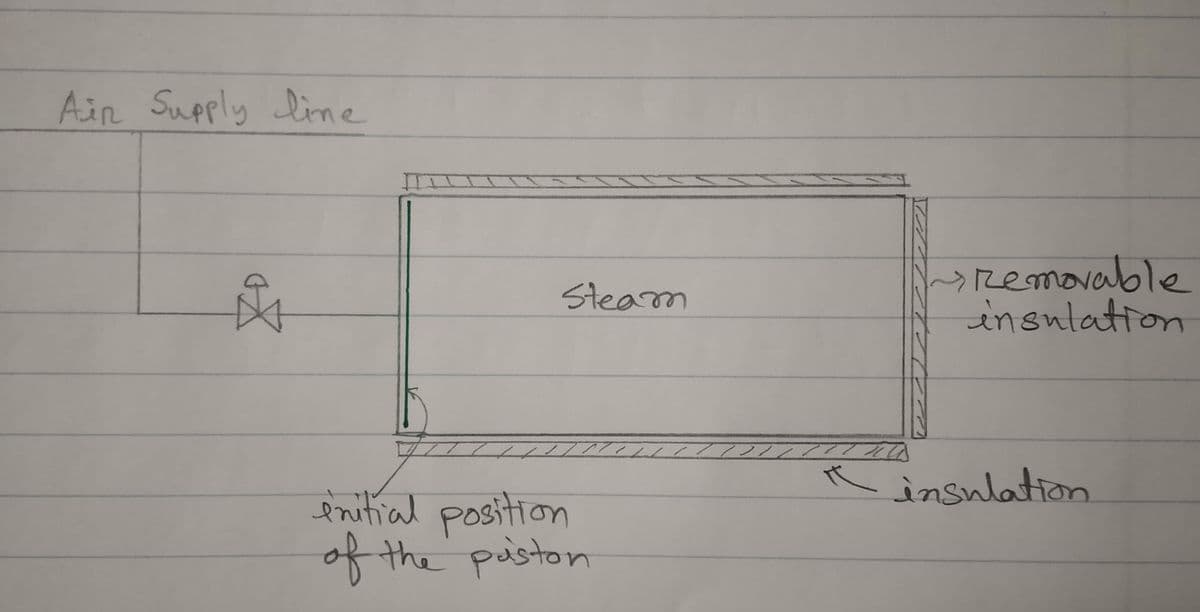
Consider a well-insulated piston-cylinder assembly. The volume of the cylinder is 3.258 m3. You can assume the piston to be frictionless and that it does not occupy a significant volume in the cylinder. Initially the piston is placed such that the entire volume of the cylinder is filled with steam at 100 kPa and 200°C. The cylinder is connected to a pipeline carrying air at 500 kPa and 250°C. The valve between the pipeline and the cylinder is opened slightly allowing air to enter the cylinder very slowly until the pressure in the cylinder reaches 500 kPa. The valve is then turned off. Assume air behaves like an ideal gas with a constant heat capacity of Cp=7R/2. a) What will be the final temperature of the air if the piston is nonconducting? (Note: steam is being compressed adiabatically and very slowly by means of a frictionless piston) b) Suppose the insulation pad at the bottom of the cylinder is removed and heat is transferred to the steam side to keep its temperature constant at 200°C during the filling process. What will be the final temperature of the air in this case?
Consider a well-insulated piston-cylinder assembly. The volume of the cylinder is 3.258 m3. You can assume the piston to be frictionless and that it does not occupy a significant volume in the cylinder. Initially the piston is placed such that the entire volume of the cylinder is filled with steam at 100 kPa and 200°C. The cylinder is connected to a pipeline carrying air at 500 kPa and 250°C. The valve between the pipeline and the cylinder is opened slightly allowing air to enter the cylinder very slowly until the pressure in the cylinder reaches 500 kPa. The valve is then turned off. Assume air behaves like an ideal gas with a constant heat capacity of Cp=7R/2.
a) What will be the final temperature of the air if the piston is nonconducting?
(Note: steam is being compressed adiabatically and very slowly by means of a frictionless piston)
b) Suppose the insulation pad at the bottom of the cylinder is removed and heat is
transferred to the steam side to keep its temperature constant at 200°C during the
filling process. What will be the final temperature of the air in this case?
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









