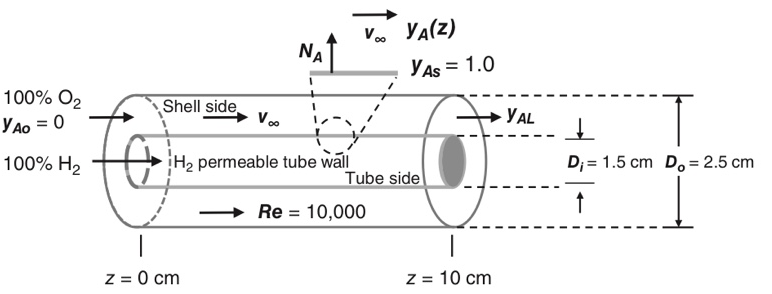
Consider the concentric tubular mass transfer device shown in the figure below. This device is designed to gradually introduce hydrogen (H2) gas (species A) into a pure oxygen gas (O2) inlet stream (species B) delivered to the shell side of the process. Pure H2 gas enters the tube side of the process. Pure O2 gas enters the shell side of the process. The tube wall material is only permeable only to H2, and not to O2. The system is designed so that the partial pressure of H2 at the gas interface and the outer surface of the inner tube wall is equal to the total system pressure, i.e. pAs P or yAs 1.0, down the entire length of the tube. In the present process, the Reynold’s number through the shell side is 10,000, the outer diameter of the inner tube is Di 1.5 cm, and the inner diameter of the outer tube is Do 2.5 cm. The tube length is L 10 cm, and the process is carried out at constant 27 C and 1.0 atm total system pressure. For the purposes of this problem, you may assume the process is dilute with respect to H2 in the O2 gas stream. Develop a material balance model, in integrated final algebraic form, to predict the outlet mole fraction of H2 from the shell side of the process, yAL. At a minimum, your model must include the following terms: Do, Di, kc, L, yAL, yAs, yAo, v∞
Consider the concentric tubular
Develop a material balance model, in integrated final algebraic form, to predict the outlet mole fraction of H2 from the shell side of the process, yAL. At a minimum, your model must include the following terms: Do, Di, kc, L, yAL, yAs, yAo, v∞.
Step by step
Solved in 3 steps









