Consider the flasks in the following diagram. 2.50 L 1.00 L 435 torr 0.260 atm What are the final partial pressures of H2 and N2 after the stopcock between the two flasks is opened? (Assume the final volume is 3.50 L.) H2 = torr N2 = torr What is the total
Consider the flasks in the following diagram. 2.50 L 1.00 L 435 torr 0.260 atm What are the final partial pressures of H2 and N2 after the stopcock between the two flasks is opened? (Assume the final volume is 3.50 L.) H2 = torr N2 = torr What is the total
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter6: The Gaseous State
Section: Chapter Questions
Problem 6.76QE
Related questions
Question
Consider the flasks in the following diagram.
|
|
||
| 2.50 L | 1.00 L | |
| 435 torr | 0.260 atm |
What are the final partial pressures of H2 and N2 after the stopcock between the two flasks is opened? (Assume the final volume is 3.50 L.)
H2 = torr
N2 = torr
What is the total pressure (in torr)?
torr
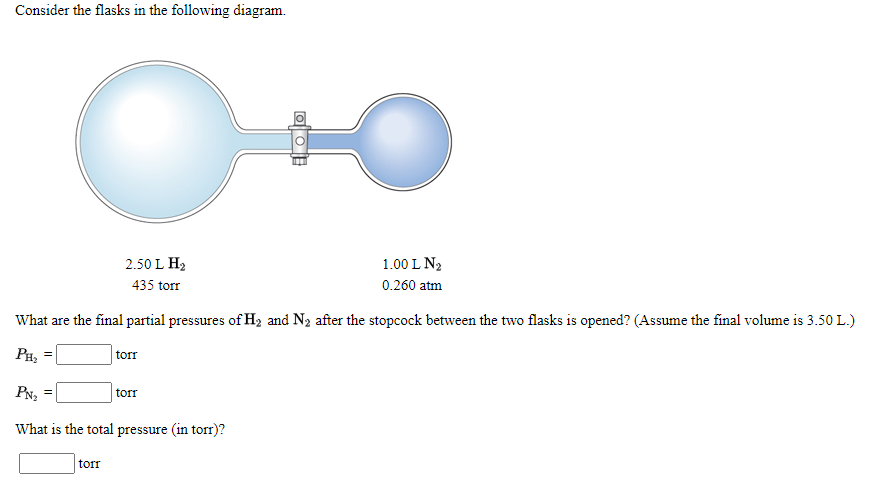
Transcribed Image Text:Consider the flasks in the following diagram.
2.50 L H2
1.00 L N2
435 torr
0.260 atm
What are the final partial pressures ofH2 and N2 after the stopcock between the two flasks is opened? (Assume the final volume is 3.50 L.)
PH,
torr
PN2
torr
What is the total pressure (in torr)?
torr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning