Consider the following reaction run at 25 °C: L (g) + Cl2 (g) = 2 ICI (g) a) A mixture at 25 °C containing I2 and Cl2 at a pressure of 0.125 atm and IC| at a pressure of 1.50 atm has DG=+1.41 kJ. Calculate the standard change in Gibbs free energy for this reaction and use the information to complete Part b) b) Fill in the blanks in the paragraph below. Write your answers in the boxes on your test template that correspond to the blanks in the paragraph. Under the specified conditions (P2 = Pcz = 0.125 atm and Pıa = 1.50 atm) at 25 °C, the value AG = +1.41 kJ indicates that this reaction will proceed in the (i) direction until a state of (ii) is reached and the value of AG = (iii) change has a (iv)_ (v)_ reaction will proceed in the (viii). (ix) value of (x). The standard free energy value, indicating that when Piz = - the %3D Pca = (vi)_ , and Pici = (vii)_ direction until AG = At that point, the standard free energy change will have a at 298 K.
Consider the following reaction run at 25 °C: L (g) + Cl2 (g) = 2 ICI (g) a) A mixture at 25 °C containing I2 and Cl2 at a pressure of 0.125 atm and IC| at a pressure of 1.50 atm has DG=+1.41 kJ. Calculate the standard change in Gibbs free energy for this reaction and use the information to complete Part b) b) Fill in the blanks in the paragraph below. Write your answers in the boxes on your test template that correspond to the blanks in the paragraph. Under the specified conditions (P2 = Pcz = 0.125 atm and Pıa = 1.50 atm) at 25 °C, the value AG = +1.41 kJ indicates that this reaction will proceed in the (i) direction until a state of (ii) is reached and the value of AG = (iii) change has a (iv)_ (v)_ reaction will proceed in the (viii). (ix) value of (x). The standard free energy value, indicating that when Piz = - the %3D Pca = (vi)_ , and Pici = (vii)_ direction until AG = At that point, the standard free energy change will have a at 298 K.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section: Chapter Questions
Problem 121QRT
Related questions
Question
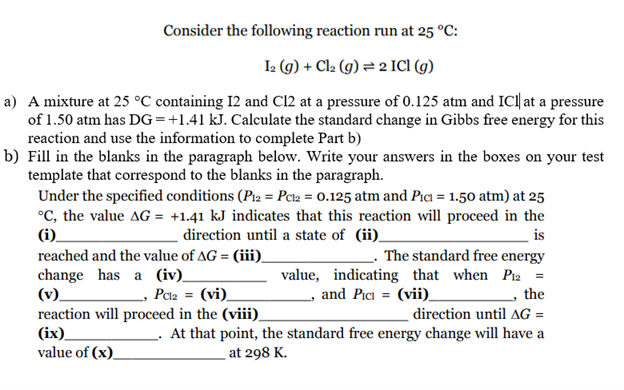
Transcribed Image Text:Consider the following reaction run at 25 °C:
Ia (9) + Cla (g) = 2 ICI (g)
a) A mixture at 25 °C containing 12 and C12 at a pressure of 0.125 atm and IC| at a pressure
of 1.50 atm has DG=+1.41 kJ. Calculate the standard change in Gibbs free energy for this
reaction and use the information to complete Part b)
b) Fill in the blanks in the paragraph below. Write your answers in the boxes on your test
template that correspond to the blanks in the paragraph.
Under the specified conditions (P12 = Pca = 0.125 atm and Pıa = 1.50 atm) at 25
°C, the value AG = +1.41 kJ indicates that this reaction will proceed in the
(i).
direction until a state of (ii)_
is
The standard free energy
value, indicating that when Piz2 =
the
reached and the value of AG = (iii)
change has a (iv).
(v).
reaction will proceed in the (viii).
(ix).
value of (x)_
- Pcla = (vi)_
, and Pici = (vii).
direction until AG =
At that point, the standard free energy change will have a
at 298 K.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax