Consider the galvanic series (shown below). From the choices provided below, please select the three metals or alloys that may galvanically.prolect brass. Your possible choices are: Cadmium, Copper, Silver, Titanium, Tin, Zine Table 16.2 The Galvank Serles Platinuns Gold Graphite Titanium Sver 316 Stainles stoel (pamive) 04 Stainles steel (pamive) Inconel (8ONi-LJC-7) (paive) Nickel (pasive) Monet (70NI-C) Copper-nickel alley a Bronzes (CV-Sn alloys) Copper Brasses (Co-Za alkoys) Inconel (active) Nickel (active) Incrcasingly inert (cathodie) Tin Lead 316 Stainles steel (tive) 04 Stalnle stee (tive) Cast iron Increasingly active (ainodie) Iron and steel Alumieum alloys Calmium Cdtcialy pre aluminum Zine Galv Ser Magdreerfagneium aloys Soervel M.G Foelana Corrosion Ergiering dedision Cegyrighe 1 by MGree ok Company Reprinted with perminin. Table 162 OJahn Wiey & Sons, Inc. All ights rerved
Consider the galvanic series (shown below). From the choices provided below, please select the three metals or alloys that may galvanically.prolect brass. Your possible choices are: Cadmium, Copper, Silver, Titanium, Tin, Zine Table 16.2 The Galvank Serles Platinuns Gold Graphite Titanium Sver 316 Stainles stoel (pamive) 04 Stainles steel (pamive) Inconel (8ONi-LJC-7) (paive) Nickel (pasive) Monet (70NI-C) Copper-nickel alley a Bronzes (CV-Sn alloys) Copper Brasses (Co-Za alkoys) Inconel (active) Nickel (active) Incrcasingly inert (cathodie) Tin Lead 316 Stainles steel (tive) 04 Stalnle stee (tive) Cast iron Increasingly active (ainodie) Iron and steel Alumieum alloys Calmium Cdtcialy pre aluminum Zine Galv Ser Magdreerfagneium aloys Soervel M.G Foelana Corrosion Ergiering dedision Cegyrighe 1 by MGree ok Company Reprinted with perminin. Table 162 OJahn Wiey & Sons, Inc. All ights rerved
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 83E: Consider a concentration cell that has both electrodes made of some metal M. Solution A in one...
Related questions
Question
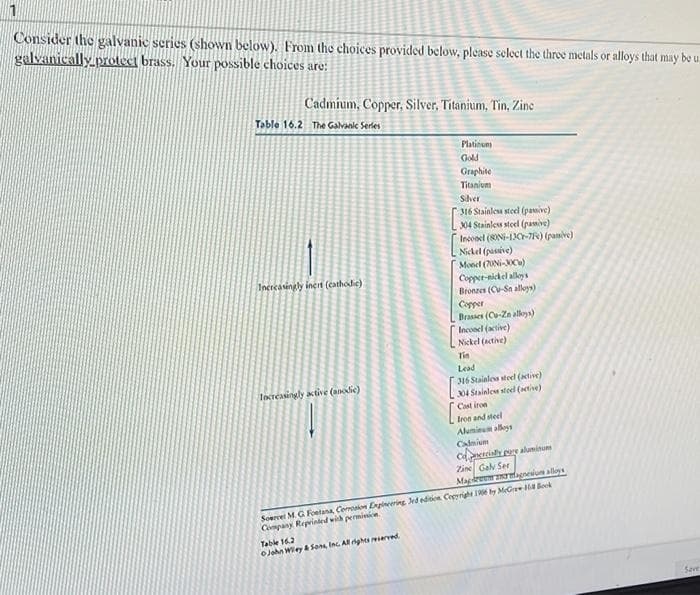
Transcribed Image Text:Consider the galvanic series (shown below). From the choices provided below, please select the three metals or alloys that may be u
galvanically protect brass. Your possible choices are:
Cadmium, Copper, Silver, Titanium, Tin, Zine
Table 16.2 The Galvank Series
Platinuns
Gold
Graphite
Titanium
Shver
316 Stainlesa stoel (pamive)
304 Stainles steel (pamive)
Inconel (8IN-1JC-7) (pamive)
Nickel (passive)
Monel (70NI-C)
Incrcasingly inert (cathodic)
Copper-nickel alloy s
Bronzes (C-Sn alloys)
Copper
Brasses (Ce-Za alkoys)
Inconel (active)
Nickel (active)
Tin
Lead
316 Stainles stee (tive)
04 Stalnle stoel (tive)
Increasingly active (aodie)
Cast iron
Iroe and steel
Aluminum alleys
Camium
Carial pere aluminum
Zine Galv Ser
Magdremfagnesium alloys
Soevei M.G Foetana Corrosion Ergineering dedition Ceeright 1E by MGre a Boek
Company Reprinled wich permivicn.
Table 16.2
OJahn Wiley Sons Inc. All ghes ered
Save
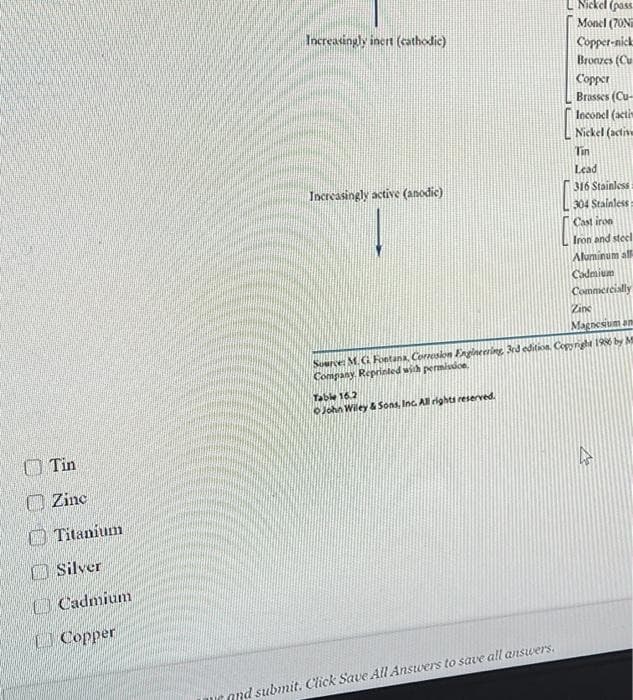
Transcribed Image Text:L Niekel (pas
Monel (70N
Increasingly inert (cathodic)
Copper-nick
Bronzes (Cu
Copper
Brasses (Cu-
Inconel (acti
Nickel (activ
Tin
Lead
Increasingly active (anodic)
316 Stainless
304 Stainless
Cast iron
Iron and steel
Aluminum al
Cadmium
Commercislly
Zine
Magnesium an
Source: M. G Fontana, Correskon Engineering 3rd edition, Copyrght 196 by M
Company. Reprinled with permissice.
Table 16.2
OJohn Wiley & Sons, Inc. All rights reserved.
Tin
O Zine
O Titanium
OSilver
Cadmium
UCopper
and submit. Click Save All Answers to save all answers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning