Consider the reaction below. Use the balanced chemical equation to fill in the blanks of the table. 3 TI + 2 N2 → TINA Tap on a table cell to enter or edit its value. # moles of #moles of # moles of N2 grams of TIN TI TI,N. 3.0 1.0 B 4.0 99.8 8.0 6.9 Color-Coding Key: Not checked Correct Incorrect Entering answer Check Answers
Consider the reaction below. Use the balanced chemical equation to fill in the blanks of the table. 3 TI + 2 N2 → TINA Tap on a table cell to enter or edit its value. # moles of #moles of # moles of N2 grams of TIN TI TI,N. 3.0 1.0 B 4.0 99.8 8.0 6.9 Color-Coding Key: Not checked Correct Incorrect Entering answer Check Answers
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 83E: Consider a concentration cell that has both electrodes made of some metal M. Solution A in one...
Related questions
Question
Please answer this asap I request you
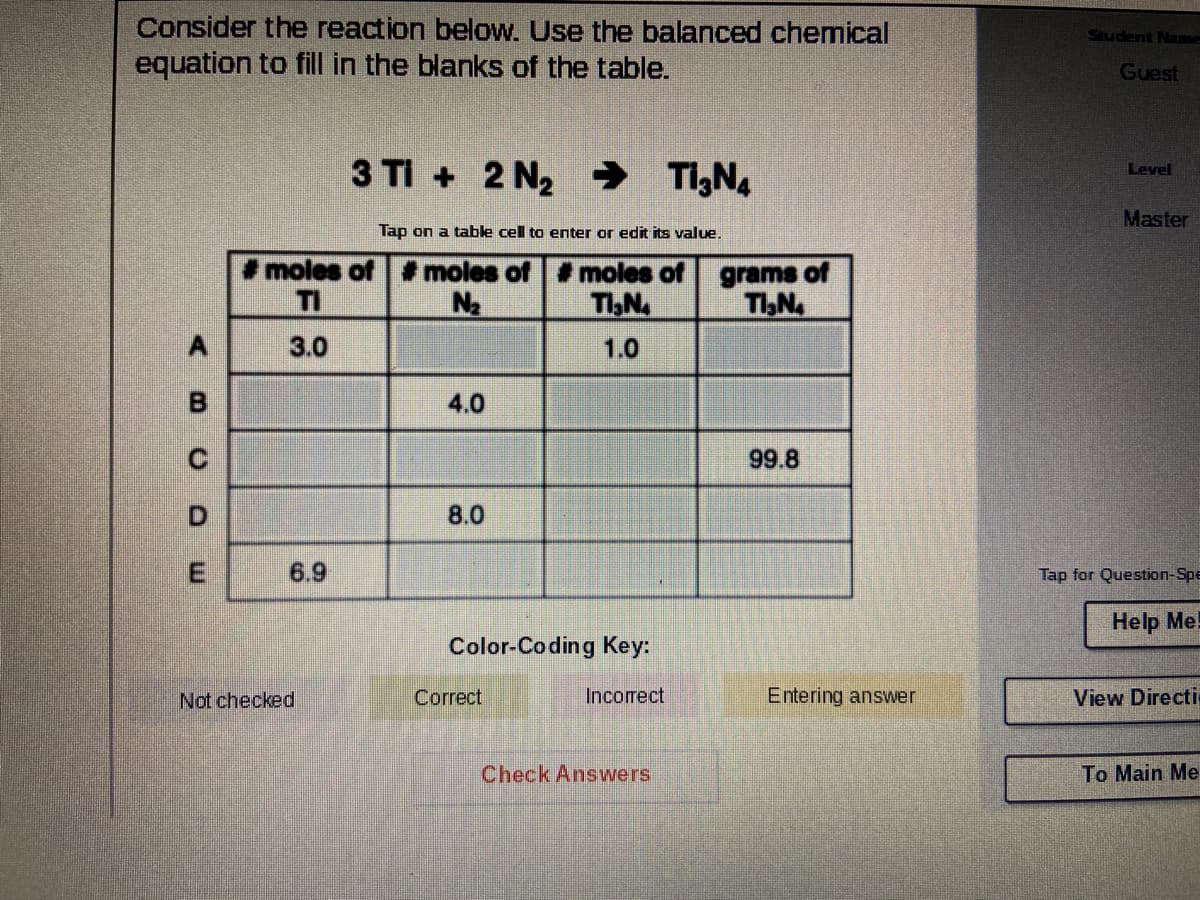
Transcribed Image Text:Consider the reaction below. Use the balanced chemical
equation to fill in the blanks of the table.
Student Name
Guest
3 TI + 2 N2 → T,N.
Level
Master
Tap on a table cell to enter or edit its value.
moles of # moles of moles of
grams of
TI,N
TI
N2
TIN
A.
3.0
1.0
4.0
99.8
8.0
6.9
Tap for Question-Spe
Help Me!
Color-Coding Key:
Not checked
Correct
Incorrect
Entering answer
View Directi
Check Answers
To Main Me
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning