Consider the titration of 50.0 mL of 0.20 M NH3 (Kp = 1.8 × 10¬³) with 0.20 M HNO3. Calculate the pH after addition of 50.0 mL of the titrant at 25 °C. Express the pH numerically. • View Available Hint(s) ? pH =
Consider the titration of 50.0 mL of 0.20 M NH3 (Kp = 1.8 × 10¬³) with 0.20 M HNO3. Calculate the pH after addition of 50.0 mL of the titrant at 25 °C. Express the pH numerically. • View Available Hint(s) ? pH =
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.85QE
Related questions
Question
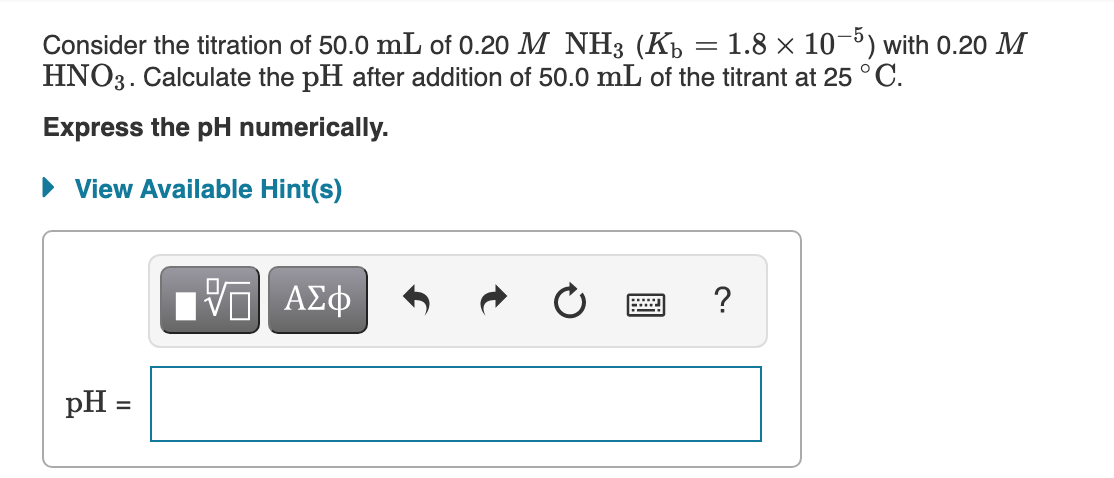
Transcribed Image Text:Consider the titration of 50.0 mL of 0.20 M NH3 (Kp = 1.8 × 10-5) with 0.20 M
HNO3. Calculate the pH after addition of 50.0 mL of the titrant at 25 ° C.
Express the pH numerically.
• View Available Hint(s)
| ΑΣφ
?
pH =
Transcribed Image Text:14
12
10
8
+ Equivalence point
6.
4
2
20.0
40.0
60.0
80.0
mL of 0.100 M NaOH added
на
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole