Consider the titration of 50.0 mL of 1.00 M CsHsN by 0.500 M HCl. For each volume of HCI added, decide which of the components is a major species after the HCI has reacted completely. K for CsHgN- 1.7 x 10. 0.00 ml HCI added no: H yes: H₂O no: CI yes: CHN nơi CSHSNH HINT: Because no HCI has been added, this solution only contains a weak base and water. See text for discussion of weak base-strong acid titrations. You are correct. Your receipt no. is 157-35117 Previous Tries Submit Answer Tries 0/45 30.00 mL HCl added no: H yes: H₂O yes: C yes: C₂H₂N yes: CH NH HINT: Assume the added strong acid neutralizes (reacts.completely with) the weak base present. You are correct. Your receipt no. Is 157- 6783 Previous Tries 100.00 ml HCl added no: H+ yes: H₂O yes: C no: CH₂N yes: CH NH HINT: Assume the added strong acid neutralizes (reacts completely with the weak base present. Determine the neutralization reaction then perfor the stoichiometry calculation. See text for discussion of weak base-strong acid titrations. You are correct. Your receipt no. is 157-1549 Previous Tries Calculate the pH at the equivalence point for this titration. Submit Answer Tries 0/45 At what volume of HCI added in this titration does the pH 5.237 Express your answer in mL and include the units in your answer.
Consider the titration of 50.0 mL of 1.00 M CsHsN by 0.500 M HCl. For each volume of HCI added, decide which of the components is a major species after the HCI has reacted completely. K for CsHgN- 1.7 x 10. 0.00 ml HCI added no: H yes: H₂O no: CI yes: CHN nơi CSHSNH HINT: Because no HCI has been added, this solution only contains a weak base and water. See text for discussion of weak base-strong acid titrations. You are correct. Your receipt no. is 157-35117 Previous Tries Submit Answer Tries 0/45 30.00 mL HCl added no: H yes: H₂O yes: C yes: C₂H₂N yes: CH NH HINT: Assume the added strong acid neutralizes (reacts.completely with) the weak base present. You are correct. Your receipt no. Is 157- 6783 Previous Tries 100.00 ml HCl added no: H+ yes: H₂O yes: C no: CH₂N yes: CH NH HINT: Assume the added strong acid neutralizes (reacts completely with the weak base present. Determine the neutralization reaction then perfor the stoichiometry calculation. See text for discussion of weak base-strong acid titrations. You are correct. Your receipt no. is 157-1549 Previous Tries Calculate the pH at the equivalence point for this titration. Submit Answer Tries 0/45 At what volume of HCI added in this titration does the pH 5.237 Express your answer in mL and include the units in your answer.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter15: Acid-base Equilibria
Section: Chapter Questions
Problem 14Q: Consider the following pH curves for 100.0 mL of two different acids with the same initial...
Related questions
Question
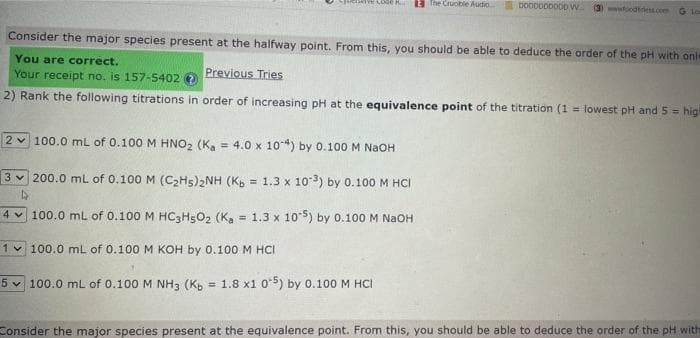
Transcribed Image Text:Consider the major species present at the halfway point. From this, you should be able to deduce the order of the pH with onl
You are correct.
Previous Tries
Your receipt no. is 157-5402 →
2) Rank the following titrations in order of increasing pH at the equivalence point of the titration (1= lowest pH and 5= higi
2 100.0 mL of 0.100 M HNO₂ (Ka 4.0 x 10-4) by 0.100 M NaOH
=
3200.0 mL of 0.100 M (C₂H5)2NH (Kb = 1.3 x 103) by 0.100 M HCI
4 100.0 mL of 0.100 M HC3H5O2 (Ka = 1.3 x 105) by 0.100 M NaOH
1 100.0 mL of 0.100 M KOH by 0.100 M HCI
The Crucible Audio Do00000000 W (3) wwwfoodtriess.com G Low
5 100.0 mL of 0.100 M NH3 (Kb = 1.8 x1 05) by 0.100 M HCI
Consider the major species present at the equivalence point. From this, you should be able to deduce the order of the pH with
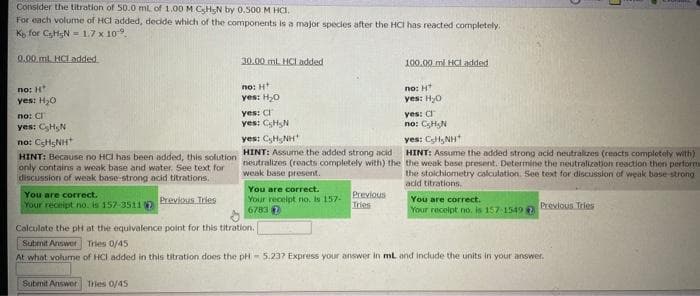
Transcribed Image Text:Consider the titration of 50.0 mL of 1.00 M CsHsN by 0.500 M HCI.
For each volume of HCI added, decide which of the components is a major species after the HCI has reacted completely.
K for CsHgN= 1.7 x 10.
0.00ml HCL added
no: H
yes: H₂O
no: C
yes: C₂HN
nơi CSHSNH
HINT: Because no HCI has been added, this solution
only contains a weak base and water. See text for
discussion of weak base-strong acid titrations.
You are correct.
Your receipt no. is 157-3511
Previous Tries
Submit Answer Tries 0/45
30.00 mL HCl added
no: H
yes: H₂O
yes: C
yes: CH N
yes: CH NH
HINT: Assume the added strong acid
neutralizes (reacts completely with) the
weak base present.
You are correct.
Your receipt no. is 157-
6783 7
Previous
Tries
100.00 ml HCI added
no: HT
yes: H₂0
yes: C
no: CHN
yes: CH₂NH
HINT: Assume the added strong acid neutralizes (reacts completely with)
the weak base present. Determine the neutralization reaction then performa
the stoichiometry calculation. See text for discussion of weak base-strong
acid titrations.
You are correct.
Your receipt no. is 157-1549 2
Previous Tries
Calculate the pH at the equivalence point for this titration.
Submit Answer Tries 0/45
At what volume of HCI added in this titration does the pH 5.237 Express your answer in mL and include the units in your answer.
E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning