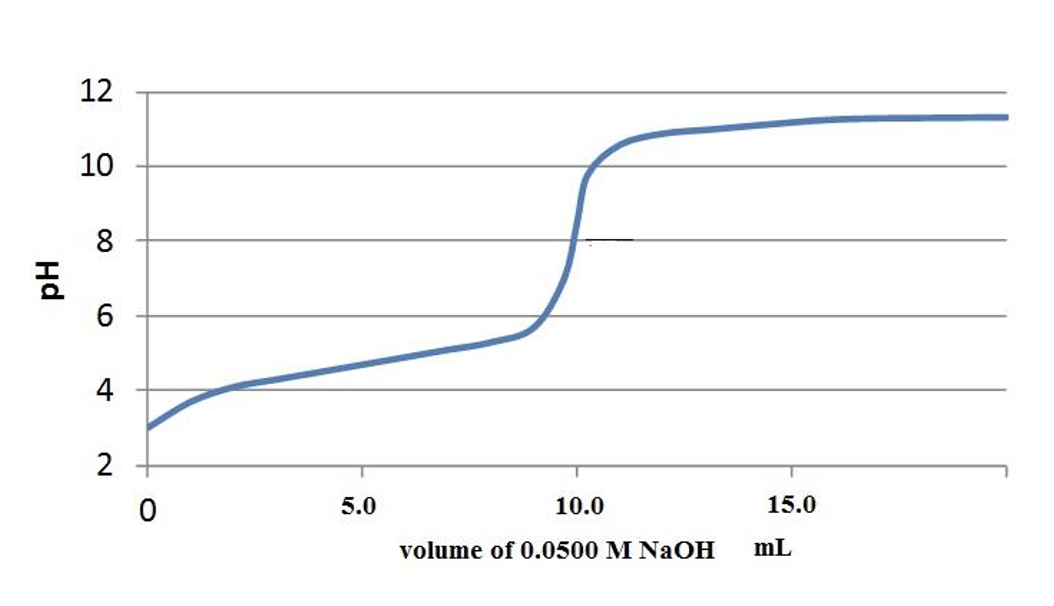
Consider the titration of the weak acid, benzoic acid, HC7H5O2. Its acid dissociation reaction is: HC7H5O2 (aq) + H2O (l) ↔ C7H5O2- (aq) + H3O+ (aq) A 25.00 mL sample of a solution of benzoic acid, concentration unknown, is titrated with 0.0500M NaOH solution. A plot of the titration is shown below. 1. what is the pKa (approximately) of benzoic acid? Its Ka? 2. on the plot, indicate the buffering region of the titration 3. From the pH at the equivalence point, estimate the pKb of benzoate ion (conjugate base to benzoic acid)______________ [Remember, at the equivalence point, C7H5O2- (aq) + H2O (l) ↔ HC7H5O2 (aq) + OH- (aq) ]
Consider the titration of the weak acid, benzoic acid, HC7H5O2. Its acid dissociation reaction is: HC7H5O2 (aq) + H2O (l) ↔ C7H5O2- (aq) + H3O+ (aq) A 25.00 mL sample of a solution of benzoic acid, concentration unknown, is titrated with 0.0500M NaOH solution. A plot of the titration is shown below. 1. what is the pKa (approximately) of benzoic acid? Its Ka? 2. on the plot, indicate the buffering region of the titration 3. From the pH at the equivalence point, estimate the pKb of benzoate ion (conjugate base to benzoic acid)______________ [Remember, at the equivalence point, C7H5O2- (aq) + H2O (l) ↔ HC7H5O2 (aq) + OH- (aq) ]
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.69PAE
Related questions
Question
Consider the titration of the weak acid, benzoic acid, HC7H5O2. Its acid dissociation reaction is:
HC7H5O2 (aq) + H2O (l) ↔ C7H5O2- (aq) + H3O+ (aq)
A 25.00 mL sample of a solution of benzoic acid, concentration unknown, is titrated with 0.0500M NaOH solution. A plot of the titration is shown below.
1. what is the pKa (approximately) of benzoic acid? Its Ka?
2. on the plot, indicate the buffering region of the titration
3. From the pH at the equivalence point, estimate the pKb of benzoate ion (conjugate base to benzoic acid)______________
[Remember, at the equivalence point, C7H5O2- (aq) + H2O (l) ↔ HC7H5O2 (aq) + OH- (aq) ]
Transcribed Image Text:12
10
8
4
5.0
10.0
15.0
volume of 0.0500 M NaOH
mL
Hd
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning