▼ Constants I Periodic Table Part A Phosphorus-32 has a half-life of 14.0 days. Starting with 2.00 g of 32P, how many grams will remain after 56.0 days? Express your answer numerically in grams. ► View Available Hint(s) ? 15. ΑΣΦ 0.062 bo g Submit Previous Answers X Incorrect; Try Again; 3 attempts remaining Your answer is the fraction of the original sample that remains. Take into account that there were initially 2.00 g present. Part B Carbon-14 has a half-life of 5730 years. In a plant fossil, you find that the ¹4C has decayed to 1/32.0 of the original amount. How long ago was this plant alive? P Pearson Contact oling Permissions
▼ Constants I Periodic Table Part A Phosphorus-32 has a half-life of 14.0 days. Starting with 2.00 g of 32P, how many grams will remain after 56.0 days? Express your answer numerically in grams. ► View Available Hint(s) ? 15. ΑΣΦ 0.062 bo g Submit Previous Answers X Incorrect; Try Again; 3 attempts remaining Your answer is the fraction of the original sample that remains. Take into account that there were initially 2.00 g present. Part B Carbon-14 has a half-life of 5730 years. In a plant fossil, you find that the ¹4C has decayed to 1/32.0 of the original amount. How long ago was this plant alive? P Pearson Contact oling Permissions
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter18: The Nucleus: A Chemist's View
Section: Chapter Questions
Problem 28E
Related questions
Question
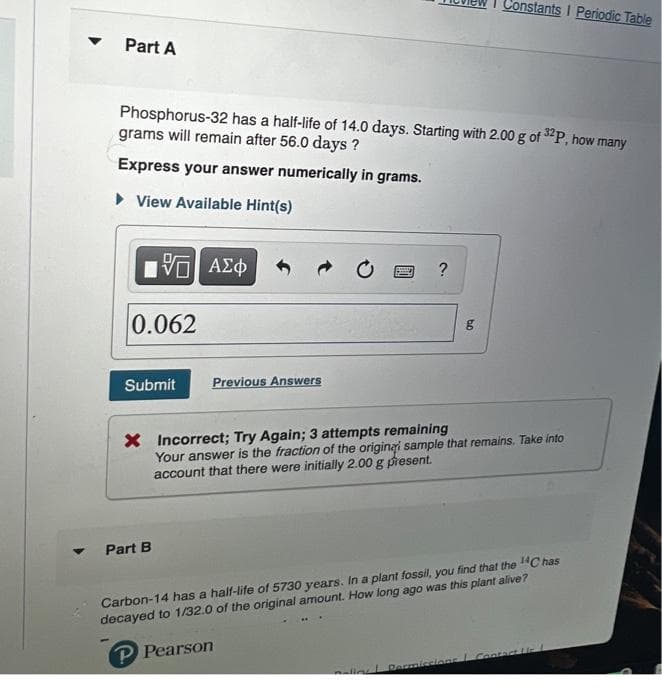
Transcribed Image Text:▼
Constants I Periodic Table
Part A
Phosphorus-32 has a half-life of 14.0 days. Starting with 2.00 g of 32P, how many
grams will remain after 56.0 days?
Express your answer numerically in grams.
► View Available Hint(s)
?
15. ΑΣΦ
0.062
bo
g
Submit
Previous Answers
X Incorrect; Try Again; 3 attempts remaining
Your answer is the fraction of the original sample that remains. Take into
account that there were initially 2.00 g present.
Part B
Carbon-14 has a half-life of 5730 years. In a plant fossil, you find that the ¹4C has
decayed to 1/32.0 of the original amount. How long ago was this plant alive?
P Pearson
Contact
Policy Permissions
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning