Constants needed for the calculations: c = 3.00 x 108 meters/second Planck's Constant (h) = 6.63 x 10-34 joules seconds nf = 2 Red Light Green Light Wavelength 656.2 486.1 (nm) Wavelength (m) ΔΕ (J) ni Blue Light 434.0 Violet Light 410.0
Constants needed for the calculations: c = 3.00 x 108 meters/second Planck's Constant (h) = 6.63 x 10-34 joules seconds nf = 2 Red Light Green Light Wavelength 656.2 486.1 (nm) Wavelength (m) ΔΕ (J) ni Blue Light 434.0 Violet Light 410.0
Chapter7: Light And Color
Section: Chapter Questions
Problem 18E: Classify each of the following lasers as to type (solid-state, gas, dye, or semiconductor), and list...
Related questions
Question
Please help with work for red light column so I can use that to complete the others, thank!
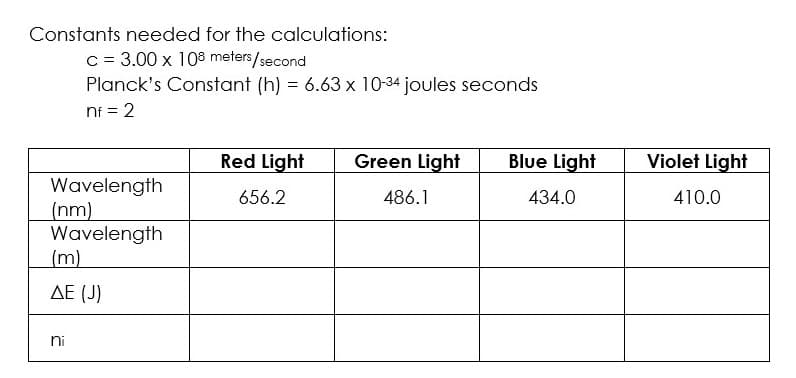
Transcribed Image Text:Constants needed for the calculations:
c = 3.00 x 108 meters/second
Planck's Constant (h) = 6.63 x 10-34 joules seconds
nf = 2
Red Light
Green Light
Wavelength
656.2
486.1
(nm)
Wavelength
(m)
ΔΕ (J)
ni
Blue Light
434.0
Violet Light
410.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning