Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.19QAP
Related questions
Question
100%
7
Transcribed Image Text:1oolS
Add-ons Help
Last edit was made 2 days ago by ERIC MATTSSON
100%
Shar
Normal text
Calibri
10
B IUA
E = E E E E - E E E X
4
Editing
5
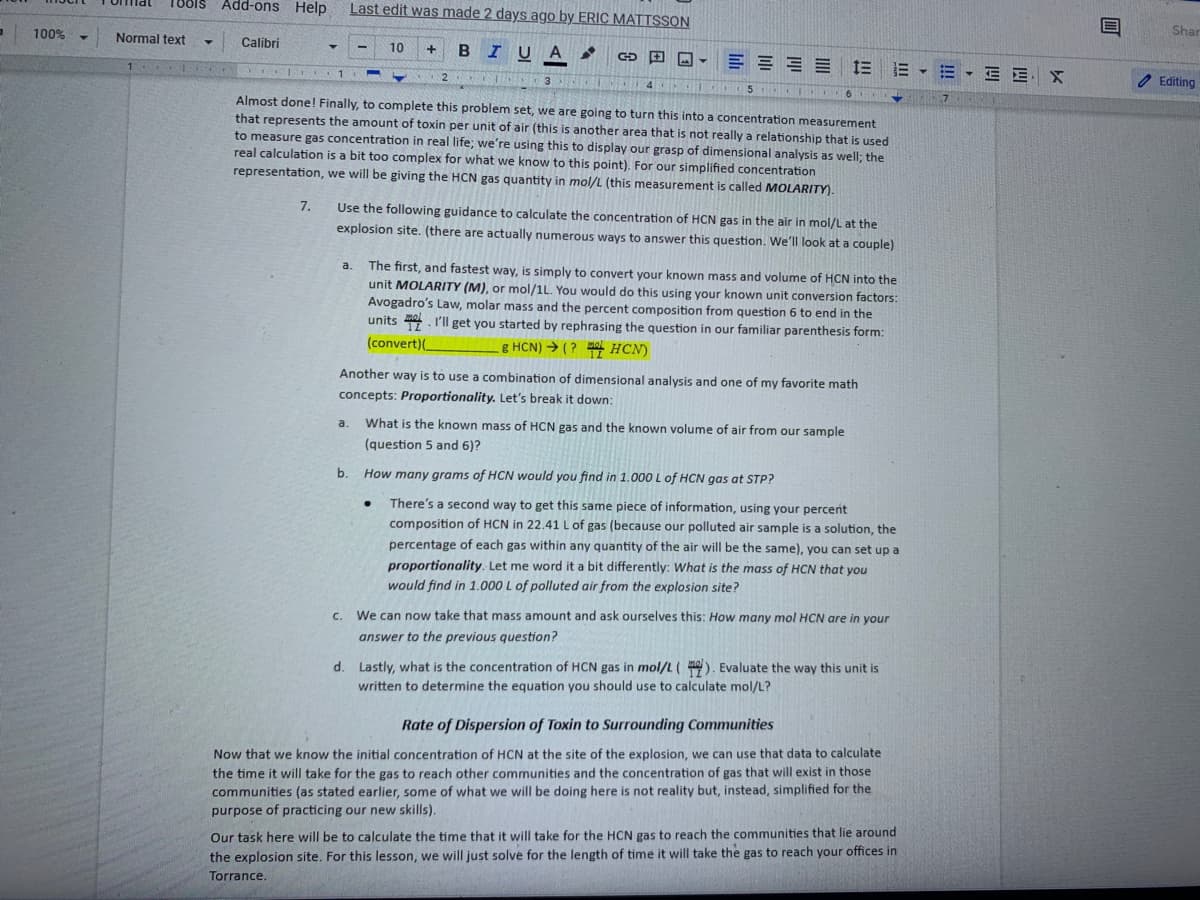
Almost done! Finally, to complete this problem set, we are going to turn this into a concentration measurement
that represents the amount of toxin per unit of air (this is another area that is not really a relationship that is used
to measure gas concentration in real life; we're using this to display our grasp of dimensional analysis as well; the
real calculation is a bit too complex for what we know to this point). For our simplified concentration
representation, we will be giving the HCN gas quantity in mol/L (this measurement is called MOLARITY).
7.
Use the following guidance to calculate the concentration of HCN gas in the air in mol/L at the
explosion site. (there are actually numerous ways to answer this question. We'll look at a couple)
The first, and fastest way, is simply to convert your known mass and volume of HCN into the
unit MOLARITY (M), or mol/1L. You would do this using your known unit conversion factors:
Avogadro's Law, molar mass and the percent composition from question 6 to end in the
units . I'll get you started by rephrasing the question in our familiar parenthesis form:
a.
(convert)(_
g HCN) → ( ? HCN)
Another way is to use a combination of dimensional analysis and one of my favorite math
concepts: Proportionality. Let's break it down:
a.
What is the known mass of HCN gas and the known volume of air from our sample
(question 5 and 6)?
b.
How many grams of HCN would you find in 1.000 L of HCN gas at STP?
There's a second way to get this same piece of information, using your percent
composition of HCN in 22.41 L of gas (because our polluted air sample is a solution, the
percentage of each gas within any quantity of the air will be the same), you can set up a
proportionality. Let me word it a bit differently: What is the mass of HCN that you
would find in 1.000 L of polluted air from the explosion site?
C.
We can now take that mass amount and ask ourselves this: How many mol HCN are in your
answer to the previous question?
d. Lastly, what is the concentration of HCN gas in mol/L(). Evaluate the way this unit is
written to determine the equation you should use to calculate mol/L?
Rate of Dispersion of Toxin to Surrounding Communities
Now that we know the initial concentration of HCN at the site of the explosion, we can use that data to calculate
the time it will take for the gas to reach other communities and the concentration of gas that will exist in those
communities (as stated earlier, some of what we will be doing here is not reality but, instead, simplified for the
purpose of practicing our new skills).
Our task here will be to calculate the time that it will take for the HCN gas to reach the communities that lie around
the explosion site. For this lesson, we will just solve for the length of time it will take the gas to reach your offices in
Torrance.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co