Current Attempt in Progress Carbon tetrachloride (CC14) was prepared by reacting 119 g of carbon disulfide and 119 g of chlorine. Calculate the percent yield if 65.0 g of CCl4 were obtained from the reaction CS2 + 3 Cl₂ → CCl4 + S₂Cl2. i % yield Want to see a relevant text example?
Current Attempt in Progress Carbon tetrachloride (CC14) was prepared by reacting 119 g of carbon disulfide and 119 g of chlorine. Calculate the percent yield if 65.0 g of CCl4 were obtained from the reaction CS2 + 3 Cl₂ → CCl4 + S₂Cl2. i % yield Want to see a relevant text example?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 100CP: he production capacity for acrylonitrile (C3H3N)in the United States is over 2 billion pounds per...
Related questions
Question
100%
Transcribed Image Text:CHRIRAD
mework
WP
NWP Assessment Player Ul Appli X
tion.wiley.com/was/ui/v2/assessment-player/index.html?launchld=d7fbf299-2b78-4aab-9fe4-25d65ce73435#/question/5
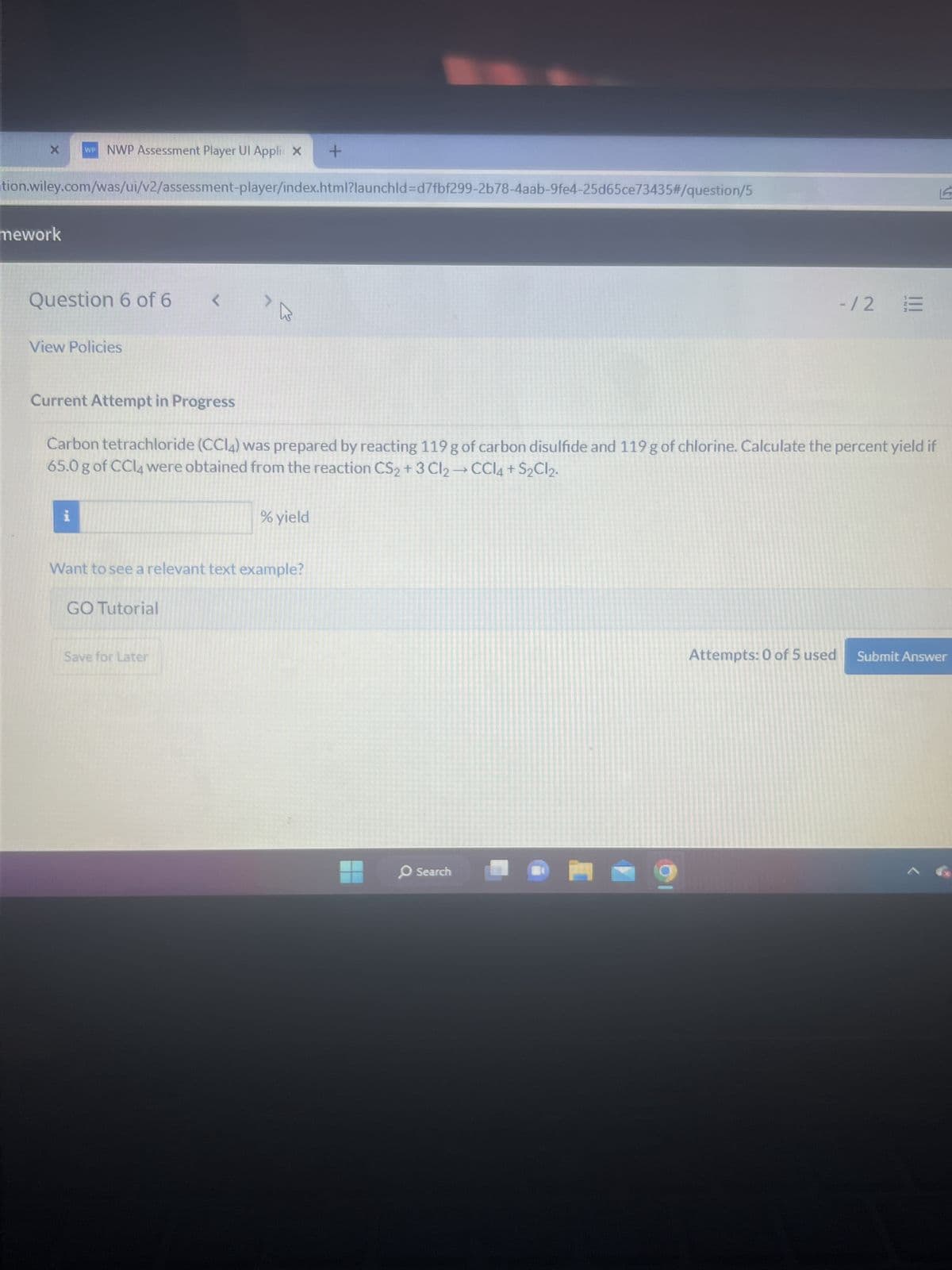
Question 6 of 6 <
View Policies
Current Attempt in Progress
i
>W
Carbon tetrachloride (CC14) was prepared by reacting 119 g of carbon disulfide and 119 g of chlorine. Calculate the percent yield if
65.0 g of CCl4 were obtained from the reaction CS₂+3 Cl₂ → CCl4 + S₂Cl2.
Save for Later
+
% yield
Want to see a relevant text example?
GO Tutorial
-/2 =
O Search
کا
Attempts: 0 of 5 used Submit Answer
Transcribed Image Text:CHRIRAD
mework
WP
NWP Assessment Player Ul Appli X
tion.wiley.com/was/ui/v2/assessment-player/index.html?launchld=d7fbf299-2b78-4aab-9fe4-25d65ce73435#/question/5
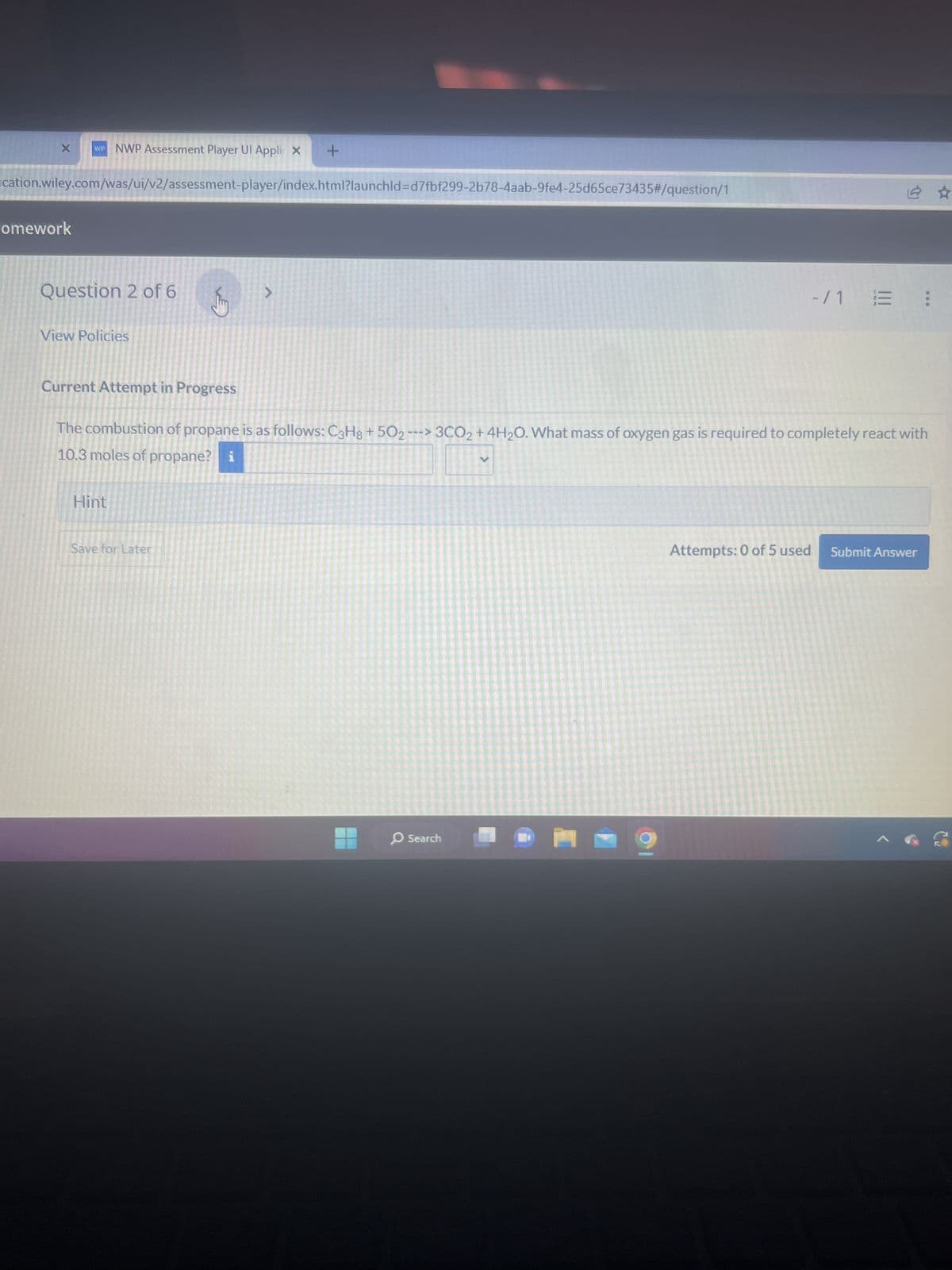
Question 6 of 6 <
View Policies
Current Attempt in Progress
i
>W
Carbon tetrachloride (CC14) was prepared by reacting 119 g of carbon disulfide and 119 g of chlorine. Calculate the percent yield if
65.0 g of CCl4 were obtained from the reaction CS₂+3 Cl₂ → CCl4 + S₂Cl2.
Save for Later
+
% yield
Want to see a relevant text example?
GO Tutorial
-/2 =
O Search
کا
Attempts: 0 of 5 used Submit Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning