Current is passed through a cell where the half-reaction that occurs at the cathode is 5e + MnO4 + 8H* - Mn2+ + 4H20 All the MnO 4 ions present in 25.0 mL of solution have been reduced after a current of 0.600 ampere has passed for 844 seconds. What was the original concentration of MnO 4 ions? O a. 1.02 x 10-1 M O b.0.21 M О с. 1.47 М O d.7.10 x 10-3 M О е. 420х 10-2 м
Current is passed through a cell where the half-reaction that occurs at the cathode is 5e + MnO4 + 8H* - Mn2+ + 4H20 All the MnO 4 ions present in 25.0 mL of solution have been reduced after a current of 0.600 ampere has passed for 844 seconds. What was the original concentration of MnO 4 ions? O a. 1.02 x 10-1 M O b.0.21 M О с. 1.47 М O d.7.10 x 10-3 M О е. 420х 10-2 м
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 135CWP: Consider a galvanic cell based on the following half-reactions: a. What is the expected cell...
Related questions
Question
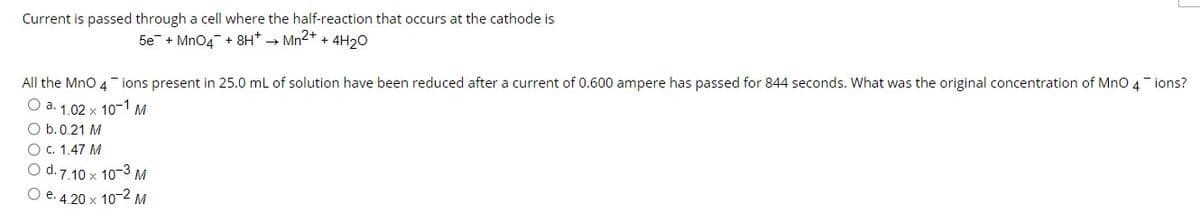
Transcribed Image Text:Current is passed through a cell where the half-reaction that occurs at the cathode is
5e + Mn04 + 8H* → Mn2+ + 4H20
All the MnO 4 ions present in 25.0 mL of solution have been reduced after a current of 0.600 ampere has passed for 844 seconds. What was the original concentration of MnO 4 ions?
O a. 1.02 x 10-1 M
O b.0.21 M
O C. 1.47 M
O d. 7.10 x 10-3 M
O e. 4.20 x 10-2 M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning