c=vΧλ. (7.1) Since the product of v and A is a constant, they have a reciprocal relationship radiation with a high frequency has a short wavelength, and vice versa and Because the atom can change its energy only by integer multiples of hv, the small- est change occurs when an atom in a given energy state changes to an adjacent state, that is, when An = 1: AE = hv (7.2)
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Problem A student uses a microwave oven to heat a meal. The wavelength of the radiation is 1.20 cm. What is the energy of one photon of this microwave radiation?
Plan We know λ in centimeters (1.20 cm) so we convert to meters, find the frequency with as shoen, and then find the energy of one photon with as shown.
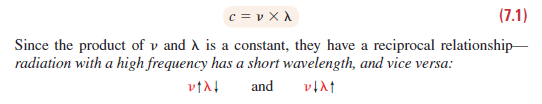
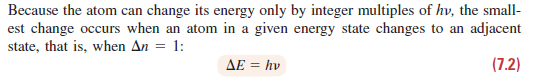
Step by step
Solved in 3 steps


