d. The student observed that, addition of Barium chloride solution to an aqueous solutions of samples D and E produced a white precipitate. Write the possible reactions which leads to the formation of the precipitate. Identify the ions present in samples D and E based on the solubility of the precipitate in dilute HCl. precipitation titrations. F. Why is neutral medium or slightly alkaline medium more suitable for Mohr’s method and What are the color changes at the end point?
I NEED SOLUTIONS OF Q D and F PLEASE
8. A student Ahmed was given five samples of simple salts labeled as A,B,C,D and E and he was
asked to analyze systematically and report the acid and basic radical present in each. Answer
the following questions based on the analysis.
a. Write the name of any two preliminary tests that Ahmed has to definitely perform.
b. Name a reagent which Ahmed can use to distinguish between a chloride, bromide and iodide
ion containing salt. Explain the reactions involved between each halide ion and the reagent.
c. Write any two safety measures he can follow while heating a solution in a test tube.
d. The student observed that, addition of Barium chloride solution to an aqueous solutions of
samples D and E produced a white precipitate. Write the possible reactions which leads to the
formation of the precipitate. Identify the ions present in samples D and E based on the solubility
of the precipitate in dilute HCl.
precipitation titrations.
F. Why is neutral medium or slightly alkaline medium more suitable for Mohr’s method and
What are the color changes at the end point?
Part D
The salt samples D and E can either contain sulfite ion or sulfate ion. If the samples consist of sulfite ion, the precipitate disappears on the addition of HCl. On the other hand, if the samples consist of sulfate ion, the precipitate shall remain insoluble and persistant in the solution even after the addition of HCl. If M represents the cation in the salt samples D and E, the chemical equations which show precipitation can be written as follows-
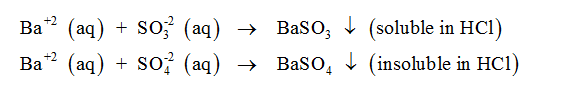
Step by step
Solved in 2 steps with 1 images


