Data: Antoine coefficients (P in mmHg, Tin K, log to base e): Margules parameters: 1. a) Calculate the Henry's Law constant for dilute methanol in water at 1 atmosphere: i) assuming ideal solution behaviour; using the Margules Coefficients provided to account for the non-ideality of the solution. Compare your answers with the value roughly obtained from the gradient in the x-y diagram below. Ym (molefraction of methanol in vapour) Methanol: A = 18.588, B = 3626.6, C = -34.29 Water: A = 18.304, B = 3816.4, C = -46.13 Ethanol: A = 18.9119, B = 3803.98, C = -41.68 b) Now repeat part a) but this time to determine the Henry's Law constant for dilute water in methanol at p=1 atm. Again, compare your results to the constant roughly obtained from the x-y diagram. 0.8 0.6 Methanol-Water: Awm = 0.6174, Amw = 0.7279 Ethanol-Water: Awe = 0.7917, Aew = 1.6366 0.4 0.2 x-y diagram for methanol/water at 1 atm 0.2 0.6 0.4 Xm (molefraction of methanol in liquid) 0.8
Data: Antoine coefficients (P in mmHg, Tin K, log to base e): Margules parameters: 1. a) Calculate the Henry's Law constant for dilute methanol in water at 1 atmosphere: i) assuming ideal solution behaviour; using the Margules Coefficients provided to account for the non-ideality of the solution. Compare your answers with the value roughly obtained from the gradient in the x-y diagram below. Ym (molefraction of methanol in vapour) Methanol: A = 18.588, B = 3626.6, C = -34.29 Water: A = 18.304, B = 3816.4, C = -46.13 Ethanol: A = 18.9119, B = 3803.98, C = -41.68 b) Now repeat part a) but this time to determine the Henry's Law constant for dilute water in methanol at p=1 atm. Again, compare your results to the constant roughly obtained from the x-y diagram. 0.8 0.6 Methanol-Water: Awm = 0.6174, Amw = 0.7279 Ethanol-Water: Awe = 0.7917, Aew = 1.6366 0.4 0.2 x-y diagram for methanol/water at 1 atm 0.2 0.6 0.4 Xm (molefraction of methanol in liquid) 0.8
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.22QAP
Related questions
Question
Feedback - Q1
Most of you managed this well once you realised that for Henry’s Law, the temperature should be approximated as the boiling temperature of the bulk component. This allowed the pure component vapour pressure of the dilute component to be evaluated.
For the comparison with the x-y diagram, the determination of the initial gradient was approximate, but the value you found should certainly have corresponded more closely to the non-ideal Henry’s Law constant. This comment applies both to part (a) and part (b).
Answer to Q1: a) (i) 3.48 atm; (ii) 7.21 atm, b) (i) 0.242 atm; (ii) 0.449 atm
PART 1a) (ii) PLEASE STEPS ON HOW TO REACH ANSWER
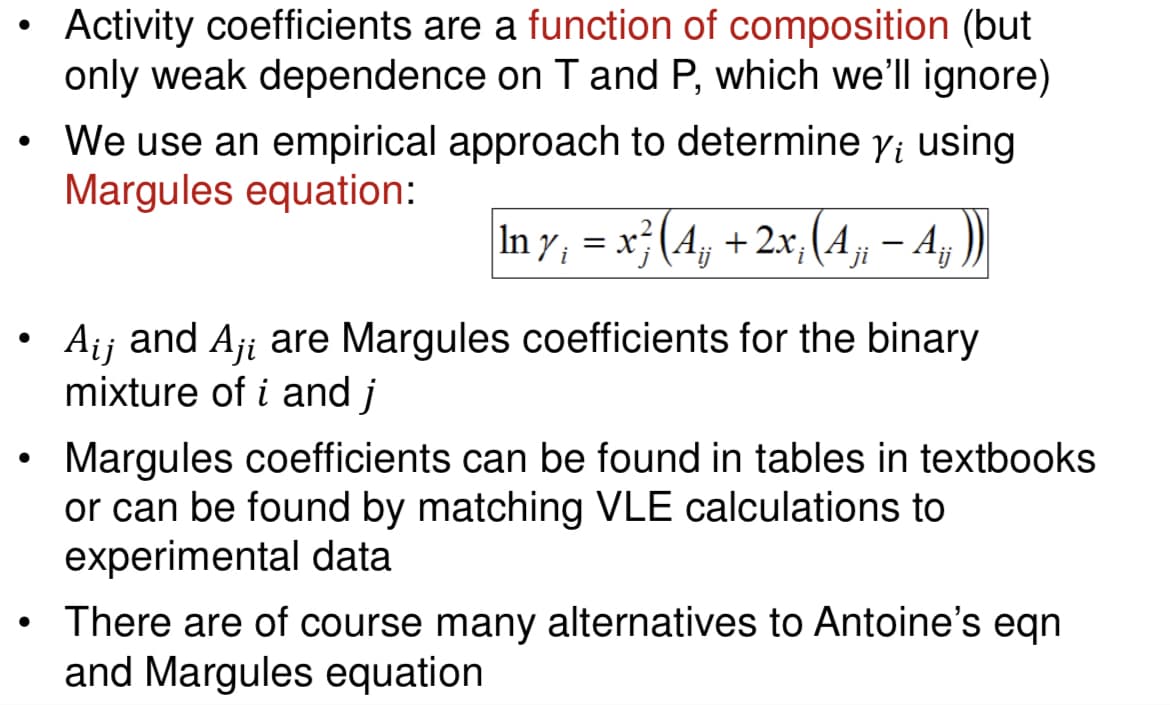
Transcribed Image Text:Activity coefficients are a function of composition (but
only weak dependence on T and P, which we'll ignore)
We use an empirical approach to determine y; using
Margules equation:
In y¡ = x² (A¡¡ + 2x; (A¡¡ — Aj
A₁j and Aji are Margules coefficients for the binary
mixture of i and j
Margules coefficients can be found in tables in textbooks
or can be found by matching VLE calculations to
experimental data
There are of course many alternatives to Antoine's eqn
and Margules equation
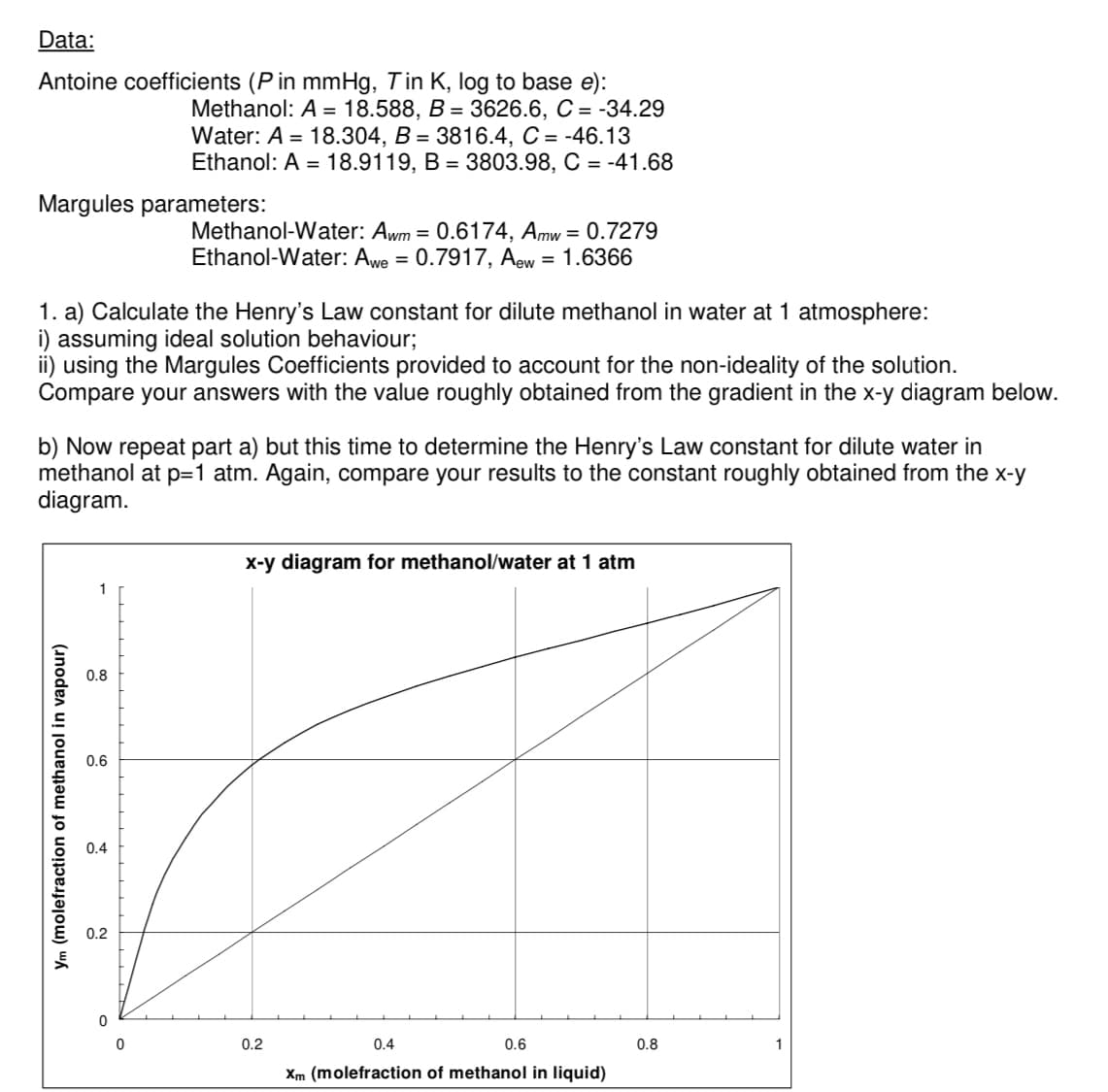
Transcribed Image Text:Data:
Antoine coefficients (P in mmHg, Tin K, log to base e):
Margules parameters:
1. a) Calculate the Henry's Law constant for dilute methanol in water at 1 atmosphere:
i) assuming ideal solution behaviour;
using the Margules Coefficients provided to account for the non-ideality of the solution.
Compare your answers with the value roughly obtained from the gradient in the x-y diagram below.
Ym (molefraction of methanol in vapour)
Methanol: A = 18.588, B = 3626.6, C = -34.29
Water: A = 18.304, B = 3816.4, C = -46.13
Ethanol: A = 18.9119, B = 3803.98, C = -41.68
b) Now repeat part a) but this time to determine the Henry's Law constant for dilute water in
methanol at p=1 atm. Again, compare your results to the constant roughly obtained from the x-y
diagram.
0.8
0.6
Methanol-Water: Awm = 0.6174, Amw = 0.7279
Ethanol-Water: Awe = 0.7917, Aew = 1.6366
0.4
0.2
x-y diagram for methanol/water at 1 atm
0.2
0.6
0.4
Xm (molefraction of methanol in liquid)
0.8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
