Data: (Remember to write correct units!!) 1st Type of Metal: Aluminnum I52.5g 10.19 Mass of Water (in calorimeter) 42.14 27°C 2nd Type of Metal:CORPER 71.2g 10:1g Mass of Water (in calorimeter)69.19 27°C ऽ४°८ . Mass of Cups & Water Mass of Cups & Water Mass of Cups Mass of Cups Initial Temp. of Water Use for #1 below Initial Temp. of Water *Final Temp of Water *Final Temp of Water 91g 33° Use for #2 88°0 1:89 32°C Mass of metal Mass of metal Initial Temp. Hot Metal Initial Temp. Hot Metal below *Final Temp. Metal (in calorimeter) *Final Temp. Metal (in calorimeter) *= same temp. Results: 1. Calculate the heat gained by the water (lost by the substance) in the calorimeter using the equation in the Introduction. Remember to write the units!! Q for water using 2nd Metal Q for water using 1st Metal Qwater = mwater X (Tf-Ti)water X Cp w Qwater = mwater x (Tf-Ti)water x Cp water water 2. Calculate the specific heat of the substance using the answer from number 1 and the equation in the Introduction. Now, Cp is your unknown since you're using Q from #1. Cp for 2nd Metal Cp of 1st Metal Qsubst = msubst x (Ti-Tf)subat x Cp su Qsubst Qsubst = mgubst x (Ti-Tf)subst x Cp Qsubst subst subst Cp = Cp = mubat X (Ti-Tf) subst mubst X (Tj-Tf) sut subst Cp = Jig- C Cp = J/g - C 3 sf 3 sf
Data: (Remember to write correct units!!) 1st Type of Metal: Aluminnum I52.5g 10.19 Mass of Water (in calorimeter) 42.14 27°C 2nd Type of Metal:CORPER 71.2g 10:1g Mass of Water (in calorimeter)69.19 27°C ऽ४°८ . Mass of Cups & Water Mass of Cups & Water Mass of Cups Mass of Cups Initial Temp. of Water Use for #1 below Initial Temp. of Water *Final Temp of Water *Final Temp of Water 91g 33° Use for #2 88°0 1:89 32°C Mass of metal Mass of metal Initial Temp. Hot Metal Initial Temp. Hot Metal below *Final Temp. Metal (in calorimeter) *Final Temp. Metal (in calorimeter) *= same temp. Results: 1. Calculate the heat gained by the water (lost by the substance) in the calorimeter using the equation in the Introduction. Remember to write the units!! Q for water using 2nd Metal Q for water using 1st Metal Qwater = mwater X (Tf-Ti)water X Cp w Qwater = mwater x (Tf-Ti)water x Cp water water 2. Calculate the specific heat of the substance using the answer from number 1 and the equation in the Introduction. Now, Cp is your unknown since you're using Q from #1. Cp for 2nd Metal Cp of 1st Metal Qsubst = msubst x (Ti-Tf)subat x Cp su Qsubst Qsubst = mgubst x (Ti-Tf)subst x Cp Qsubst subst subst Cp = Cp = mubat X (Ti-Tf) subst mubst X (Tj-Tf) sut subst Cp = Jig- C Cp = J/g - C 3 sf 3 sf
Chapter5: Temperature And Heat
Section: Chapter Questions
Problem 8Q: (¦ Indicates a review question, which means it requires only a basic understanding of the material...
Related questions
Question
Need help answering questions 1 and 2
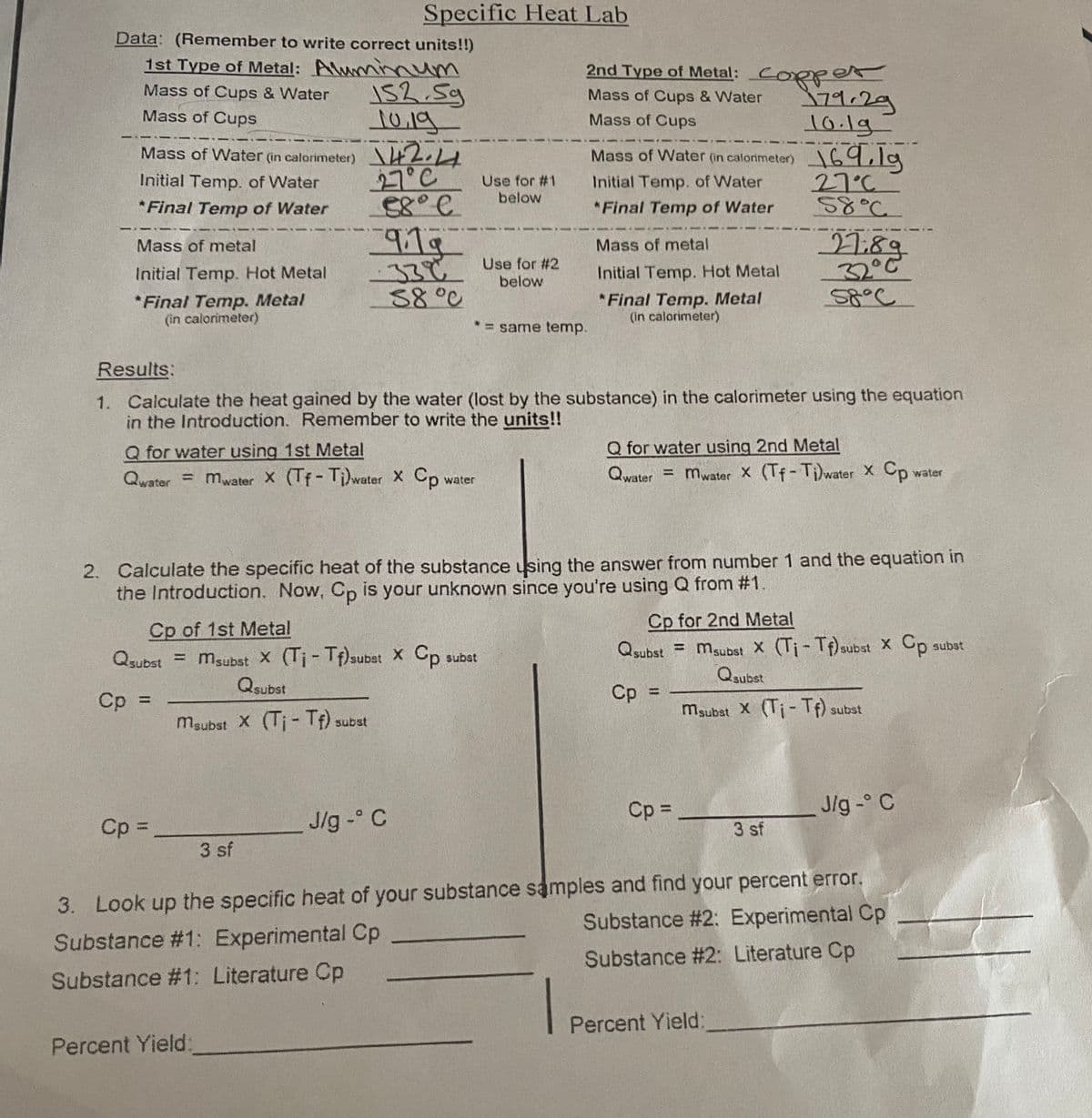
Transcribed Image Text:Specific Heat Lab
Data: (Remember to write correct units!!)
1st Type of Metal: Aluminnum
2nd Type of Metal: CORper
152.5g
10.19
Mass of Water (in calorimeter) 2.14
27°C
Mass of Cups & Water
79.29
10.1g
Mass of Cups & Water
Mass of Cups
Mass of Cups
I69,1g
27°C
ऽ४°८.
Mass of Water (in calorimeter)
Initial Temp. of Water
Use for #1
Initial Temp. of Water
below
*Final Temp of Water
*Final Temp of Water
919
27:89
32°C
Mass of metal
Mass of metal
33¢ Use for #2
below
Initial Temp. Hot Metal
Initial Temp. Hot Metal
88°0
*Final Temp. Metal
(in calorimeter)
*Final Temp. Metal
(in calorimeter)
* = same temp.
Results:
1. Calculate the heat gained by the water (lost by the substance) in the calorimeter using the equation
in the Introduction. Remember to write the units!!
Q for water using 1st Metal
Q for water using 2nd Metal
Qwater = mwater x (Tf-Ti)water Xx Cp
water
Qwater = mwater X (Tf- Ti)water X Cp
water
2. Calculate the specific heat of the substance using the answer from number 1 and the equation in
the Introduction. Now, Cp is your unknown since you're using Q from #1.
Cp for 2nd Metal
Cp of 1st Metal
Qsubst = msubst X (Ti-Tf) subst X Cp subst
Qsubst
%3D
Qsubst = msubst X (Tj- Tf)subat X Cp
Qsubst
%3D
subst
1sqns
Cp
%3D
maubat X (Ti- If) subst
maubst X (Ti- If) subst
Cp =
J/g - C
Cp =
Jlg - C
3 sf
3 sf
3. Look up the specific heat of your substance samples and find your percent error.
Substance # 2: Experimental Cp
Substance #1: Experimental Cp
Substance #2: Literature Cp
Substance #1: Literature Cp
|
Percent Yield:
Percent Yield:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College



College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning